Abstract
There is an urgent need to develop means of ex situ biobanking and biopreserving corals and other marine organisms whose habitats have been compromised by climate change and other anthropogenic stressors. To optimize laboratory growth of soft corals in a way that could also benefit industry (e.g., aquarium trade), three culture systems were tested herein with Sarcophyton glaucum: (1) a recirculating aquaculture system (RAS) without exogenous biological input (RAS−B), (2) a RAS with “live” rocks and an exogenous food supply (RAS+B), and (3) a simple flow-through system (FTS) featuring partially filtered natural seawater. In each system, the effects of two levels of photosynthetically active radiation (100 or 200 μmol quanta m−2 s−1) and flow velocity (5 or 15 cm s−1) were assessed, and a number of soft coral response variables were measured. All cultured corals survived the multi-month incubation, yet those of the RAS−B grew slowly and even paled; however, once they were fed (RAS−B modified to RAS+B), their pigmentation increased, and their oral discs readily expanded. Light had a more pronounced effect in the RAS−B system, while flow affected certain coral response variables in the FTS tanks; there were few effects of light or flow in the RAS+B system, potentially highlighting the importance of heterotrophy. Unlike the ceramic pedestals of the FTS, those of the RAS+B did not regularly become biofouled by algae. In concert with the aforementioned physiological findings, we therefore recommend RAS+B systems as a superior means of biopreservating and biobanking soft corals.
Similar content being viewed by others
Introduction
Certain soft corals and all reef-building corals form associations (collectively termed “holobionts”) with symbiotic dinoflagellates and bacteria; these microbes are critical to the physiological function and survival of their animal hosts1−3. Although the mutualistic dinoflagellates translocate photosynthetically fixed carbon into host cytoplasm, corals and other endosymbiotic anthozoans nevertheless rely on heterotrophy, as well, for nourishment4−5. These mutualistic associations consequently span a bridge between benthic and planktonic food webs and consequently play critical roles as primary and secondary producers6.
The culture of alcyonacean soft corals (Cnidaria: Anthozoa: Octocorallia: Alcyonacea)- notably Sarcophyton, Sinularia, and Lobophyton- has advanced rapidly in recent decades given the needs to (1) explore the impacts of environmental change on their physiological performance and (2) develop a sustainable supply for both the marine cosmeceutical and aquarium trade industries1,7,8. Many cultured soft corals used in natural product research rely on natural seawater flow-through systems (FTS)9,10; however, natural product yields may differ from those of wild populations11. Other studies have used recirculating aquaculture systems (RAS) in which seawater quality parameters can be readily and automatically modulated by a variety of microprocessor-based dosing systems12−14. While biological factors, such as “live rocks” (typically dead coral skeleton encrusted by crustose coralline algae) that accumulate rich microbial flora15,16 and heterotrophic feeding4−5,17, have been explored more recently, a systemic comparison across different types of culture systems has not yet been achieved for soft corals.
Light intensity and flow velocity have been examined in numerous soft coral experiments7,17−19. The former is especially important for soft corals harboring dinoflagellates, whose photosynthetic performance can directly or indirectly affect the physiology and growth of their hosts20,21. Light has also been shown to affect not only growth, but also secondary metabolite production, in symbiotic soft corals19. With respect to flow, soft corals alter their morphologies in response to changes in seawater velocity18, and seawater flow promotes material exchange and metabolism (thereby affecting metabolite yield). However, only single-factor experiments have been conducted with soft corals, despite the well-documented interaction between irradiance and water flow in hard corals like Galaxea fascicularis22. Thus, research focused on the interaction of light and flow on soft coral physiology is needed.
Measurements of morphology and growth in soft corals are more difficult compared to stony corals23; the latter consist of a large amount of calcium carbonate (exoskeleton) covered by a thin veneer of live tissue. Alcyonacean soft corals, in contrast, are mostly fleshy, with only small amounts of calcium carbonate (sclerite) skeleton. Shape is instead maintained by hydrostatic pressure from water pumped into a canal system. Such hydroskeletons change quickly in size and shape in response to environmental shifts. Therefore, it is difficult to accurately measure their size and growth23, though oral disc diameter (ODD), stalk diameter, colony height, and wet and dry colony weight have all been assessed in prior works24,25. In general, ash weight and ash-free dry weight (AFDW) are thought to represent skeleton and organic matter content, respectively25.
Soft corals of the genus Sarcophyton are abundant on many coral reefs in the Indo-Pacific Ocean, and particularly Taiwan6. Sarcophyton colonies are mushroom-shaped, with (1) fleshy stalks elevating the polyp-bearing oral discs and (2) sclerites in the interior fleshy tissues of the colony. Sarcophyton glaucum (Quoy & Gaimard, 1833) is a suspension feeder that lives in mutualistic association with phototrophic dinoflagellates (family Symbiodiniaceae), as well as an array of other microbes. It has been cultured in FTS to investigate natural product production9 and in RAS to assess the effects of light intensity and flow velocity on its physiology17,20,26,27. Given the recent recommendation to develop the capacity to biobank and biopreserve corals ex situ28, a rigorous comparison of soft coral performance across different culture systems is of need.
Herein we aimed to study the effect of three different types of culture systems—a RAS with no microbial flora or exogenously supplied food [RAS minus (−) additional biological input = RAS−B], a RAS without a protein skimmer supplemented with live rocks and a supplemental phytoplankton solution fed corals [RAS plus ( +) additional biological input = RAS+B], and a traditional FTS- on the physiological performance of S. glaucum (Fig. 1). Within each culture system, two levels of photosynthetically active radiation (PAR; 100 or 200 μmol quanta m−2 s−1) and flow velocity (5 or 15 cm s−1) were administered, and several coral physiological response variables were measured to assess performance: colony color, height, base diameter, growth rate, ODD, and percent (%) organic weight (i.e., AFDW). We hypothesized that the physiological performance of the corals would be superior in the RAS+B system due to the (1) potential for heterotrophy and (2) the fact that feeding was carried out in separate feeding tanks (which would potentially limit macroalgal growth in the culture tanks).
Results
Water quality
The pH, concentrations of Ca2+and Mg2+, and carbonate hardness/alkalinity (KH) were significantly higher in RAS−B and RAS+B than in FTS (Table 1). Levels of ammonia, nitrite, nitrate, and phosphate in the three systems remained below detectable levels (< 0.2 mg L−1) during the entire experimental period (data not shown). PAR and flow did not deviate significantly over time (p > 0.10) and remained close to their target values (see details in “Methods”).
Coral health and growth
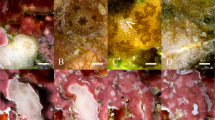
Although all fragments in the three culture systems were mushroom-shaped (Fig. 1B–D), many soft coral fragments cultured in the RAS−B system appeared unhealthy (Fig. 1B); polyps were not always extended, and neither their buoyant weight [BW; Fig. 2A; one-way ANOVA effect of time (for this and all following tests in this paragraph), F = 1.77, p = 0.134] nor their ODD (Fig. 2B; F = 0.0629, p = 0.8023) increased significantly over time. They also paled significantly over this period when pooling across all light × flow treatments (Fig. 2C; F = 53.1, p < 0.0001), though the color decrease of RAS−B corals of the two low-light treatments [high-light + low flow (Fig. 2D-1) and high-light + high flow (Fig. 2D-2)] was not statistically significant. After changing to the RAS+B system (see Fig. 1C for representative fragment images.), their ODD enlarged (Fig. 2B; F = 9.54, p < 0.01), and their pigmentation increased (Fig. 2C; F = 32.8, p < 0.0001). Green, filamentous algae tended to overgrow the pedestals in the FTS (Fig. 1D), and neither buoyant weight (Fig. 2A; p = 0.635) nor ODD (Fig. 2B; p = 0.120) increased significantly between culture days 110 and 170. FTS fragment color actually decreased over this same period (Fig. 2C), though the global temporal difference was not statistically significant (p = 0.204; see also Fig. 2D for temporal changes for each of the four light × flow interaction groups.).
Violin plots depicting temporal variation in buoyant weight (A), oral disc diameter (ODD: B), and fragment color (C) of soft corals over time in the three culture systems—recirculating aquarium system (RAS) without additional biological input (RAS−B; red), RAS with exogenous food supply and “live” rocks (RAS+B; green), and a flow-through system (FTS) featuring partially filtered seawater (blue)—as well as interaction plots of raw color data (D). It should be re-emphasized that RAS−B tanks were converted to RAS+B ones after day-84. Error bars spanning mean values at each sampling time represent standard error, asterisks in (D) denote Tukey’s honestly significant differences (p < 0.05) between RAS+B and FTS corals, and lowercase letters in (D) denote temporal differences (Tukey’s HSD, p < 0.05) in color for corals of the RAS−B culture system. RAS+B vs. FTS differences in (D) are instead denoted by asterisks (*).
In the principal components analysis (PCA) on correlations (Fig. 3), the first two axes captured nearly 60% of the variation in the dataset, and the primary coordinate (x) axis featured ODD change (eigenvalue = 0.63) and color change (0.55) as the dominant positive loading factors, with AFDW as the dominant negative one (− 0.53). The secondary (y) axis was primarily defined by the negative relationship between the SGR (0.44) and the color change (− 0.48). There was evident separation between RAS−B and RAS+B samples in the PCA, and a multivariate ANOVA (MANOVA) + canonical correlation analysis (CCA) verified this (Wilks’ lambda of culture treatment effect = 16.6, p < 0.001). A more detailed explanation on how the individual treatments (light × flow) affected these soft coral response variables can be found in Fig. 2D, Table 2 (statistically significant findings only), the online supplemental results (OSR), Supplementary Table S1 (the full repeated-measures ANOVA model), and Supplementary Figs. S1 and S2. Briefly, there were more significant effects of light on response variables measured in corals of the RAS−B [e.g., ODD growth (Supplementary Fig. S2G) and color change (Fig. 2D, Supplementary Fig. S2M)]. Flow more significantly affected the response of soft corals cultured in the FTS, particularly the AFDW (Supplementary Fig. S2L), which was higher at low flow rates. In contrast, neither light nor flow affected the SGR (Supplementary Fig. S2E) or ODD growth (Supplementary Fig. S2H) of RAS+B corals. As an exception, the color increase was higher for RAS+B corals cultured at the higher light level (Supplementary Fig. S2N), though no significant post-hoc differences were noted.
Principal components analysis of correlations across standardized soft coral physiological response variable data. Please note that specific growth rate (SGR), color change, and oral disc diameter (ODD) all reflect rates or changes over time (day−1, final–initial, and % change day−1, respectively); final values were instead incorporated for percent (%) organic weight (i.e., AFDW), which was only measured once. There was a clear inverse relationship between % ODD change day-1 and AFDW.
Discussion
All soft corals in the three culture systems survived, even those cultured for 170 days; this actually represents the longest experimental culture of soft corals (only 30 days–5 months in other studies14,20). However, corals grew slowly, and tended to become paler, in the RAS−B system; upon feeding (RAS−B modified to RAS+B), they regained pigmentation, and their ODD enlarged. Furthermore, unlike the ceramic pedestals of the FTS, those of the RAS+B did not regularly become biofouled by algae. This may not only be due to the use of synthetic seawater, in which nutrients were not present, but also because RAS+B coral feeding was undertaken in a separate tank. We therefore avoided the eutrophication associated with feeding experiments carried out in RAS12, in which algae may bloom and smother corals; when other studies carried out feeding directly in the RAS17,26, such high nutrient loads were generated that soft coral growth was ultimately thwarted. We therefore recommend to those seeking to culture soft corals over long-term timescales to use RAS and feed their organisms in separate tanks.
Fed corals demonstrate lower SGR than starved ones17. Herein the SGR of the fed corals in RAS+B was lower than in RAS−B corals for one (HLLF) of the four light × flow interaction groups; however, RAS+B ODD increased more rapidly than in the other two culture systems. The fact that the % increase in ODD (but not AFDW) was higher in RAS+B corals is likely due to heterotrophy7. For instance, fed colonies of the symbiotic temperate coral Astrangia poculata exhibited significantly greater photosynthetic efficiency than starved conspecifics29.
It should be noted that SGR and ODD data gave conflicting results: RAS−B corals grew faster when looking at the former response variable, with ODD change revealing that RAS+B corals grew faster. Given the issues raised in the Introduction with using the BW technique with predominantly soft-bodied organisms, we argue herein that soft coral biologists carefully consider its validity; perhaps the easily measured ODD is a superior, alternative metric for soft coral growth23,24,26. Indeed, others have suggested that BW data may be spurious since soft coral tissues contain a significant amount of water, meaning that the density of some colonies may be similar to that of pure seawater25. Regardless, the SGR documented herein were comparable to, or even higher than, those reported in other studies of soft corals (Table 3), despite their very small starting sizes. The average annual ODD growth rate was 1.13 cm year−1 in the three culture systems, similar to the linear growth rate of 1.0 cm year−1 of Sarcophyton (0.5–4.9 cm colony diameter) on the Great Barrier Reef24. Although our growth rates were higher than those reported in other aquarium studies, it is worth noting that, when comparing husbandry to in situ data, soft corals grew faster on the reef in the lone study that made this comparison26; it will be critical, then, to gather field data from our Taiwanese field sites to ensure that aquarium growth rates are comparable to in situ ones.
Unlike RAS−B and FTS corals, RAS+B specimens darkened over the duration of the experiment; this reflects either an increase in Symbiodiniaceae density or pigmentation within their cells (or both) and may indicate that starved endosymbionts of the RAS−B in particular were nitrogen limited (especially given the virtual absence of nitrates and nitrites in the seawater)5. However, whether the presumed influx of nitrogen and other nutrients occurred via feeding on the exogenously supplied phytoplankton feed (direct feeding)4, feeding on the microbial flora released from the live rocks (indirect feeding), or some other mechanism remains to be determined. It is worth noting that the presence of live rock was associated with higher coral survival rates and Fv/Fm values, as well as fewer bleached specimens in hard coral studies15, and we advocate continued research on the role of microbiology in marine animal health and husbandry1,3,7,8.
Organic weight (AFDW) is a reliable metric for normalizing soft coral data25, particularly when inter-species comparisons are made, and it is thought to better reflect the proportion of fleshy coral tissue. AFDW was only significantly affected by flow rate in the FTS, and percentages documented herein (26–33%) were similar to those reported for congenerics in situ (29%)24 and higher than those of cultured conspecifics (20–29%)17. On the other hand, S. glaucum values showed much larger variation (~ 10 to 55%) in a prior work that also compared different culture conditions and systems26.
Light intensity affected the ODD in the RAS−B (100 > 200 μmol quanta m−2 s−1), and both the ODD and AFDW were affected by flow in the FTS. Water flow reduces the diffusive boundary layer around the coral and influences the supply and uptake of dissolved gasses, nutrients, and food, as well as the removal of sediments and metabolic waste products; collectively, then, higher flow rates should benefit the health and growth of corals22, and such has been demonstrated in symbiotic soft corals24. Herein, though, corals generally grew faster at the lower of the two flow rates; perhaps exposure to constantly high flow rates of 15 cm s−1 results in stress to the polyps or actually sweeps food past too quickly for particles to be effectively captured.
Light more significantly affected growth (as ODD) in corals of the RAS−B. This is likely because these corals were dependent on autotrophy alone for nutrition. In contrast, there were few effects of light intensity or flow velocity in corals of the RAS+B, potentially suggesting that exogenous food supply was sufficient for coral growth; had it not been, the corals may have been more sensitive to changes in their abiotic environment. In the future, it will be worthwhile to determine whether fed soft corals obtain relatively more energy and nutrition from heterotrophy than autotrophy; it can only be stated with the data in hand that fed corals generally outperformed starved ones from assessment of the response variables measured.
Conclusions
We demonstrated that light and flow effects on soft coral physiology are culture system-dependent. Given that (1) soft corals are mostly fleshly and (2) ODD measurements can be made in only several seconds (versus minutes for buoyant weighing), we advocate assessing both ODD and the BW-based SGR in future studies of soft corals, especially Sarcophyton. Soft corals cultured in RAS+B featuring live rocks and an exogenous food supply tended to grow more quickly and presented darker pigmentation. Furthermore, by feeding these corals in a separate tank, macroalgal biofouling was virtually eliminated from the RAS+B husbandry tanks. We therefore recommend the future culture of soft corals in RAS+B systems and advocate that similar such experimental marine animal husbandry approaches be conducted elsewhere (and with additional species), especially given the recently identified need to optimize aquarium husbandry for biobanking/biopreserving marine organisms whose habitats have been compromised by climate change and other anthropogenic stressors28.
Methods
Animal material
Three S. glaucum colonies (ODD = ~ 30 cm) were collected under Kenting National Park permit 1570001572 (to TYF) at depths of 7–8 m outside the inlet of Taiwan’s third nuclear power plant (21° 57′ 15.7" N, 120° 45′ 21.2" E) in Nanwan Bay, Southern Taiwan. Colonies, which were at least 4–5 m apart, were identified in situ by assessment of their morphological characteristics6, quarantined in the husbandry facility of the National Museum of Marine Biology and Aquarium for a week, and acclimated in a 200-L flow-through tank characterized by the following conditions: natural seawater filtered to 5 μm, temperature = 26 ± 1 °C (mean ± standard error for this and all other error terms unless stated otherwise), salinity = 35 ± 1, and PAR = 100 ± 1 μmol quanta m−2 s−1 (light–dark cycle of 12 h/12 h). After this initial acclimation period, a sterilized scalpel was used to cut fragments (~ 10 mm in diameter and ~ 0.3 g BW) along the edge of the oral disc. Each of the three parent colonies produced 81 fragments, and absorbable polyglycolic acid stitches (VISORB, 1/0)30 were used to attach them to 2.7-cm, etched, T-shaped ceramic pedestals (average BW = 5.167 g); these stitches have been shown to be associated with shorter recovery and attachment times than the more commonly used rubber bands14,20. The 243 fragments were placed in a 200-L tank under the same conditions as above for recovery and attachment for 2 weeks, and all survived the preparatory processes (Fig. 1A). Of these, 108 were used in the RAS−B, and 72 of these were carried over into the RAS+B. A separate 108 were used in the FTS. All three culture systems are described in detail below. Since 72 of the 216 total fragments were analyzed in a repeated measures fashion (RAS−B modified to RAS+B), a total of 288 measurements were made for the non-destructive response variables described below.
Culture systems
RAS−B
The RAS−B included synthetic seawater: Red Sea salt (Red Sea Aquatics, Ltd.) mixed with reverse osmosis (RO) water (no additional microbial flora). In order to ensure consistent water quality, all six experimental tanks (each 60 × 35 × 20 cm; Fig. 1A) were connected in series to a 240-L “life support” tank (120 × 45 × 45 cm), which contained a 0.2-mm filter bag, an automatic Mato-2009 RO bucket (Autoaqua, Taiwan), a protein skimmer (JNS, CO2, Taiwan), a zeolite drum (JNS, ZR-2), a primary pump (Mr. Aqua, 6000 L/H, Taiwan), a titration system (Johnlen, CS072A-1, Taiwan; for measuring alkalinity [as KH] and concentrations of Ca2+ nd Mg2+), a heater (ISTA, 350W, USA), and a chiller (Resun, C-1000 p, China; 26 ± 1 °C). The salinity was maintained at 35 using a Mato-100P osmoregulator (Autoaqua) that automatically compensated for evaporative water loss by adding fresh RO water. At the beginning of the experiment, 108 fragments were randomly placed across the 6 experimental tanks (n = 18 fragments/tank), where they were then cultured for 84 days. At the end of this period, 36 fragments were randomly selected and stored in a − 20 °C freezer for analysis of organic matter (AFDW; described below).
RAS+B
The remaining 72 fragments were left in the six tanks, and the protein skimmer was removed to keep dissolved and particulate organic material in the synthetic seawater column; it was hypothesized that doing so would enhance the biological activity in the system and potentially benefit the remaining corals12. Live rocks (50 kg) were also added because, similarly, it was hypothesized that doing so could increase the growth and color of the soft corals (color paled over the first 84 days in the RAS−B treatment; see Fig. 2B,C). The addition of live rock, an effective biofilter, can improve nutrient cycling and shift microbial communities towards a more typical seawater assemblage15−16. Thus, the RAS−B was modified to a RAS+B, and the 60-day RAS+B vs. FTS (see below) experiment began on the 110th day.
In addition to the features listed above, corals of the RAS+B culture system were fed with a reef phytoplankton solution (Seachem, USA) containing concentrated microalgae (Thalassiosira weissflogii, Isochrysis sp., & Nannochloropsis sp.) once every 3 days. After the lights were turned off, fragments were moved into an independent, 20-L feeding tank (60 × 35 × 20 cm) with bubble stones in the four corners (to allow for even water mixing) and a heater that maintained the temperature at 26 °C. After 30 min, the polyps and tentacles extended, and 3 mL of the feeding solution were added. After 4 h, the fragments were removed from the feeding tank, rinsed with filtered seawater, and returned to the experimental tanks. It was hypothesized that, by feeding in a separate tank, the elevation in nutrient levels and macroalgal biomass associated with the feeding process would be avoided.
FTS
For these tanks (n = 6), natural seawater was first pumped in from the nearby ocean (Houwan Bay), filtered through sand (50 μm) and passed through 30-ton coral reef mesocosm tanks (described previously31) in which seawater temperature was maintained at 26 ± 1 °C. After filtering sequentially (100, 50, 5 μm), the seawater then entered the experimental tanks. The water flow-through (tank volume h−1) was 10 L h−1, and there was no “life support” tank. The 108 coral fragments were transferred from the acclimation tanks (where they had acclimated for 110 days) to the FTS tanks at the same time as the RAS−B were converted to RAS+B, and the FTS vs. RAS+B comparison was carried out over 60 days (culture days 110–117; see Fig. 2).
Water quality
Both the RAS−B and RAS+B systems were connected to a 240-L life support tank (315 L in total), and partial synthetic seawater in the RAS−B and RAS+B was changed weekly (30 L; ~ 10% of the volume of the entire culture system). For all three culture systems, concentrations of nutrients (nitrate, nitrite, phosphate, and ammonia), calcium (Ca2+), magnesium (Mg2+), carbonate hardness/alkalinity (KH), and pH of the three systems were measured weekly (Salifert Profi Test, Holland).
Light and flow treatments
A PVC plate was placed in the center of each of the six tanks within each of the three culture systems (Fig. 1A), and LED lights (Illumagic, ComboRay G2, Taiwan) were programmed to administer 100 μmol quanta m−2 s−1 (low light) to one half of each divided tank and 200 μmol quanta m−2 s−1 to the other (n = 36 experimental areas across the 18 tanks, each at a 12-h/12-h light/dark cycle). Light levels were chosen based on levels used in prior studies that were associated with relatively high soft coral growth rates17,19−21,26−27. Each experimental tank also contained a flow motor (Maxspect, GP-03, China), and half of the six tanks were exposed to a flow of 5 cm s−1, with the remaining three at a higher flow of 15 cm s−1. As for the PAR levels employed, these flow rates were set based on those that were associated with relatively high soft coral growth in prior studies17,19−21,26−27.
Thus, there were four treatments in triplicate within each of three culture systems: high light/low flow (HLLF), high light/high flow (HLHF), low light/low flow (LLLF), and low light/high flow (LLHF). The low/high light intensity averaged 105 ± 0.15/206 ± 0.25 μmol quanta m−2 s−1, and the low/high flow velocity averaged 4.6 ± 0.06/15.7 ± 0.16 cm s−1, as measured by light (Li-Cor, LI-193SA, USA) and flow (Kenek, GR20/GR3T-2-20 N, South Korea) meters, respectively. For RAS−B and RAS+B, 9 and 6 soft coral fragments were analyzed in each of the 12 experimental areas (2 areas for each of the 6 experimental tanks), resulting in a total of 108 and 72 fragments for the light × flow experiment, respectively. For the FTS, 9 fragments were used, resulting in a total of 108 fragments.
BW and SGR
The weights of the coral fragments were measured by a buoyant weighting technique on a Mettler Toledo AB204 balance (precision = 0.1 mg; USA). A glass beaker containing filtered seawater (26 ± 0.5 °C and salinity of 35) and a thermostatic bath were placed under the electronic scale, and the coral fragments were suspended on fishing line under the electronic scale for buoyant weight measurements. Before each measurement, the surface of the coral pedestal was lightly brushed with a toothbrush to remove algae. RAS−B fragment weights were measured every 3 weeks, while RAS+B and FTS fragments were weighed biweekly. The SGR (day−1) was calculated as: (lnWf − lnWi)/Δt, where lnWi and lnWf represented the natural logarithms of the coral fragment buoyant weights (technically buoyant mass, g, but regularly referred to as “weight” in virtually all publications) at the beginning and the end of the experiment, respectively, and ∆t represented the duration in days. We generally multiplied these data by 100 in most figures. Although we have shown the raw BW data for each culture system (Fig. 2A), we generally prioritized the SGR in our discussion since, unlike raw BW data, it accounts for potential starting size differences across corals.
ODD
The mean diameters ((length + width)/2) of the oral discs were measured at night (when tentacles had retracted) with vernier calipers (readable to 1 mm) at times = 0 and 84 days for RAS−B. A % change in ODD over this time period was calculated, since, as with BW, a relative rate of change theoretically better accounts for starting size differences across soft corals [as well as the fact that culture durations differed between RAS−B (84 days) and RAS+B and FTS (70 days)]; for this reason, temporal differences in raw ODD across all four treatments have not been depicted for each culture system (data pooled across treatments for each culture system can instead be seen in Fig. 2B, with rates of change found in all following figures). For FTS and RAS+B, measurements were made on culture days 110 and 170, with the % change in ODD also calculated. ODD % change day−1 has generally been shortened to “ODD growth” throughout the article, though, for some correlation-based analyses (see below.) raw ODD values were instead used.
Height and base diameter
The height and mean base diameter ((length + width)/2) of each colony were measured at night when the polyps were retracted with vernier calipers (as above) on culture day 170 (RAS+B and FTS only).
AFDW
Coral fragments (n = 36, 72, nd 108 for RAS−B, RAS+B, and FTS, respectively) were removed from the pedestals and dried in a vacuum freeze dryer (Xian Toption Lyophilizer, China) for 48 h. The dry weight was measured, after which the samples were incinerated at 450 °C in an MF-30 muffle furnace (Hipoint, Taiwan) for 2 h to calculate the inorganic weight. AFDW was calculated (as a proxy for % organic weight) as (dry weight-inorganic weight)/dry weight × 100.
Color score
Coral fragments were photographed with a fixed light source (5500 K, LED) in a 40 × 40 × 40 cm studio at the beginning and end of the experiment using a digital camera (Olympus, Tough TG-5). Based on CoralWatch’s “Coral Health Chart”32, fragments were scored along the E1 to E6 axis, and the changes in color scores (final assessment level-initial), rather than color itself, were assessed in the statistical tests outlined below to eliminate bias due to certain corals beginning experiments at slightly paler levels than others. That being said, we did plot raw color values over time for each treatment × culture system interaction group (Fig. 2D) to highlight differential effects of treatment on color changes for RAS+B and FTS corals, since, in some cases, starting pigmentation levels were similar.
Statistical analyses
Data were first tested for normality (Shapiro–Wilk’s test of residuals) and equal variance (Levene’s test), and, since seawater quality parameters did not conform to these assumptions, they were analyzed by non-parametric Kruskal–Wallis tests (on ranks) to determine the effect of culture system on each parameter. Dunn's multiple comparisons post-hoc tests were performed to compare individual culture system means. Because the RAS−B and RAS+B experimental stages were not independent, they were treated as repeated measures (two-way) in the statistical analysis of the physiological response variables for (1) BW (raw values and raw increases per day), (2) SGR (% change day−1), (3) % change in ODD day−1, (4) organic weight (AFDW; final time only), and (5) color. For these analyses, both the temporal change (excluding AFDW) and the final values were assessed separately. For those two parameters (colony height and base diameter) assessed only in corals of the latter two stages, RAS+B and FTS, the more traditional 3-way ANOVA (culture system × light × flow) was instead carried out since these two culture systems were fully independent. For two-way, repeated measures ANOVAs, tank was nested within light (df = 1) × flow (df = 1), whereas for three-way ANOVA, tank was nested within culture condition (df = 1 since RAS−B data were excluded from these analyses) × light × flow. Tukey’s honestly significant difference (HSD) post-hoc tests were used to compare individual mean differences among culture systems, across treatments, or in response to light and/or flow.
As a less conservative approach, one-way ANOVAs were carried out to test for the effect of culture system for each of the four light × flow treatments (Supplementary Fig. S1) for each of the following response variables: colony height, colony base diameter, SGR, ODD (final values and % changes), organic weight (final values only), and color (final values and raw changes). Similarly, two-way ANOVAs (light × flow) were carried out within each culture system for the same response variables (Supplementary Fig. S2), and one-way repeated measures ANOVAs were used to evaluate the effects of time and culture system on BW, ODD, and color (Fig. 2). Alpha levels of 0.01 and 0.05 were set a priori for ANOVAs and post-hoc tests, respectively.
To depict variation across culture systems within a multivariate framework, a principal components analysis (PCA) on correlations was carried out with standardized data from the following response variables: SGR (% day−1), ODD (% change day−1), organic weight (%; final values only), and change in color (final-initial values). A canonical correlation analysis (CCA) based on a multivariate ANOVA (MANOVA) was also carried out to uncover multivariate differences between culture systems, and Wilks’ lambda was calculated to assess statistical significance. All statistical analyses were performed using JMP (ver. 14.2).
Ethics statement
Animals involved in the experiments are not listed in CITES and were cultured in the laboratory for experimental purposes only.
References
Leal, M. C. et al. Coral aquaculture to support drug discovery. Trends Biotechnol. 31, 555–561 (2013).
Peixoto, R. S., Rosado, P. M., Leite, D. C. A., Rosado, A. S. & Bourne, D. G. Beneficial microorganisms for corals (BMC): Proposed mechanisms for coral health and resilience. Front. Microbiol. 8, 341 (2017).
van de Water, J. A. J. M., Allemand, D. & Ferrier-Pagès, C. Host-microbe interactions in octocoral holobionts—recent advances and perspectives. Microbiome 6, 64 (2018).
Piccinetti, C. C. et al. Herbivory in the soft coral Sinularia flexibilis (Alcyoniidae). Sci. Rep. 6, 22679 (2016).
Houlbrèque, F. & Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17 (2009).
Benayahu, Y., Jeng, M. S., Perkol-Finkel, S. & Dai, C. F. Soft corals (Octocorallia: Alcyonacea) from southern Taiwan. II. species diversity and distributional patterns. Zool. Stud. 43, 548–560 (2004).
Leal, M. C., Ferrier-Pagès, C., Petersen, D. & Osinga, R. Coral aquaculture: Applying scientific knowledge to ex situ production. Rev. Aquacult. 6, 1–18 (2014).
Leal, M. C. et al. Marine microorganism-invertebrate assemblages: Perspectives to solve the “supply problem” in the initial steps of drug discovery. Mar. Drugs 12, 3929–3952 (2014).
Huang, C. Y. et al. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 5, 15624 (2015).
Sang, V. T. et al. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 17, 468 (2019).
Peng, B. R. et al. Aquaculture soft coral Lobophytum crassum as a producer of anti-proliferative cembranoids. Mar. Drugs 16, 15 (2018).
Bartlett, T. C. Small scale experimental systems for coral research: Considerations, planning, and recommendations. NOAA Technical Memorandum NOS NCCOS 165 and CRCP 18, p. 68 (2013).
Farag, M. A. et al. Metabolomics reveals biotic and abiotic elicitor effects on the soft coral Sarcophyton ehrenbergi terpenoid content. Sci. Rep. 7, 648 (2017).
Mendes, C. et al. Effect of LEDs light spectrum on success of fragmentation and growth of leather coral Sarcophyton spp. Int. J. Aquacult. 7, 57–63 (2017).
Yuen, Y. S., Yamazaki, S. S., Nakamura, T., Tokuda, G. & Yamasaki, H. Effects of live rock on the reef-building coral Acropora digitifera cultured with high levels of nitrogenous compounds. Aquacult. Eng. 41, 35–43 (2009).
Bik, H. M. et al. Microbial community succession and nutrient cycling responses following perturbations of experimental saltwater aquaria. mSphere 4, e00043-19 (2019).
Costa, A. P. L. et al. The effect of mixotrophy in the ex situ culture of the soft coral Sarcophytoncf. glaucum. Aquaculture 452, 151–159 (2016).
Khalesi, M. K., Beeftink, H. H. & Wijffels, R. H. Flow-dependent growth in the zooxanthellate soft coral Sinularia flexibilis. J. Exp. Mar. Biol. Ecol. 351, 106–113 (2007).
Khalesi, M. K., Beeftink, H. H. & Wijffels, R. H. Light-dependency of growth and secondary metabolite production in the captive zooxanthellate soft coral Sinularia flexibilis. Mar. Biotechnol. 11, 488–494 (2009).
Rocha, R. J. M., Calado, R., Cartaxana, P., Furtado, J. & Serôdio, J. Photobiology and growth of leather coral Sarcophyton cf. glaucum fragments stocked under low light in a recirculated system. Aquaculture 414, 235–242 (2013).
Rocha, R. J. M., Serôdio, J., Leal, M. C., Cartaxana, P. & Calado, R. Effect of light intensity on post-fragmentation photobiological performance of the soft coral Sinularia flexibilis. Aquaculture 388, 24–29 (2013).
Schutter, M., Kranenbarg, S., Wijffels, R. H., Verreth, J. & Osinga, R. Modification of light utilization for skeletal growth by water flow in the scleractinian coral Galaxea fascicularis. Mar. Biol. 158, 769–777 (2011).
Hellstrom, H. & Benzie, J. A. H. Robustness of size measurement in soft corals. Coral Reefs 30, 787–790 (2011).
Fabricius, K. E. Slow population turnover in the soft coral genera Sinularia and Sarcophyton on mid- and outer-shelf reefs of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 126, 145–152 (1995).
Pupier, C. A., Bednarz, V. N. & Ferrier-Pagès, C. Studies with soft corals—recommendations on sample processing and normalization metrics. Front. Mar. Sci. 5, 348 (2018).
Sella, I. & Benayahu, Y. Rearing cuttings of the soft coral Sarcophyton glaucum (Octocorallia, Alcyonacea): Towards mass production in a closed seawater system. Aquacult. Res. 41, 1748–1758 (2010).
Rocha, R. J. M. et al. Development of a standardized modular system for experimental coral culture. J. World Aquacul. Soc. 46, 235–251 (2015).
Zoccola, D. et al. The World Coral Conservatory (WCC): A Noah’s ark for corals to support survival of reef ecosystems. PLoS Biol. 18(9), e3000823 (2020).
Burmester, E. M. et al. The impact of autotrophic versus heterotrophic nutritional pathways on colony health and wound recovery in corals. Ecol. Evol. 8, 10805–10816 (2018).
Meyle, J. Suture materials and suture techniques. Perio 3, 253–268 (2006).
Mayfield, A., Fan, T. Y. & Chen, C. S. Physiological acclimation to elevated temperature in a reef-building coral from an upwelling environment. Coral Reefs 32, 909–921 (2013).
Siebeck, U., Marshall, N., Klüter, A. & Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 25, 453–460 (2006).
Acknowledgements
We thank Zong-Min Ye for his comments on the culture systems. This study was funded by the Ministry of Science and Technology of Taiwan (MOST 107-2611-M-291-004).
Author information
Authors and Affiliations
Contributions
T.C.C. and T.Y.F. designed the study and performed the experiments. T.C.C., A.B.M., and T.Y.F. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare no conflict of interest, be they financial or otherwise. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, TC., Mayfield, A.B. & Fan, TY. Culture systems influence the physiological performance of the soft coral Sarcophyton glaucum. Sci Rep 10, 20200 (2020). https://doi.org/10.1038/s41598-020-77071-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77071-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.