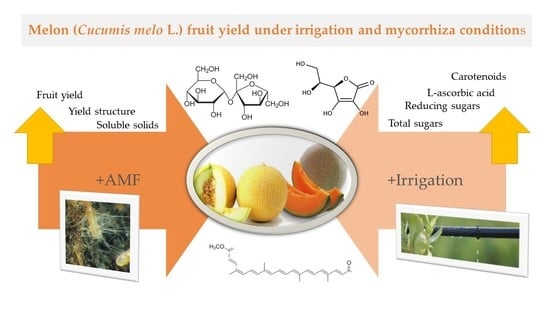
Melon (Cucumis melo L.) Fruit Yield under Irrigation and Mycorrhiza Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Place of Cultivation and Plant Material
2.2. Experimental Design
2.3. Morphological and Chemical Measurements
2.4. Chemical Analysis of Melon Fruit
2.5. Dry Matter (%)
2.6. Soluble Solids (%)
2.7. L-Ascorbic Acid (mg · 100 g−1 FM)
2.8. Total Sugars (% FM)
2.9. Reducing Sugars (% FM)
2.10. Calculation of Results
2.11. Carotenoids (mg · 100 g−1 FM)
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, S.P.; Mahapatra, B.S.; Pramanick, B.; Yadav, V.R. Effect of irrigation levels, planting methods and mulching on nutrient uptake, yield, quality, water and fertilizer productivity of field mustard (Brassica rapa L.) under sandy loam soil. Agric. Water Manag. 2021, 244, 106539. [Google Scholar] [CrossRef]
- Dharminder; Singh, R.K.; Kumar, V.; Pramanick, B.; Alsanie, W.F.; Gaber, A.; Hossain, A. The Use of Municipal Solid Waste Compost in Combination with Proper Irrigation Scheduling Influences the Productivity, Microbial Activity and Water Use Efficiency of Direct Seeded Rice. Agriculture 2021, 11, 941. [Google Scholar] [CrossRef]
- Maisiri, N.; Senzanje, A.; Rockstrom, J.; Twomlow, S.J. On farm evaluation of the effect of low cost drip irrigation on water and crop productivity compared to conventional surface irrigation system. Phys. Chem. Earth Parts A/B/C 2005, 30, 783–791. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef] [Green Version]
- Teka, T.; Kassahun, H. Characterization and evaluation of antioxidant activity of Aloe schelpei Reynolds. Drug Des. Dev. Ther. 2020, 14, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Shetty, A.A.; Magadum, S.; Managanvi, K. Vegetables as sources of antioxidants. J. Food Nutr. Disord. 2013, 2, 2. [Google Scholar] [CrossRef]
- Paris, H.S.; Amar, Z.; Lev, E. Medieval emergence of sweet melons, Cucumis melo (Cucurbitaceae). Ann. Bot. 2012, 110, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, V.K.; Gupta, J.K.; Upadhyay, P.K. Pharmacological importance of Cucumis melo L: An overview. Asian J. Pharm. Clin. Res. 2017, 10, 8–12. [Google Scholar] [CrossRef]
- Akkemik, E.; Aybek, A.; Felek, I. Effects of Cefan melon (Cucumıs melo L.) seed extracts on human erythrocyte carbonic anhydrase I-II enzymes. Appl. Ecol. Environ. Res. 2019, 17, 14699–14713. [Google Scholar] [CrossRef]
- Rabadán, A.; Nunes, M.A.; Bessada, S.M.; Pardo, J.E.; Oliveira, M.B.P.; Álvarez-Ortí, M. From by-product to the food chain: Melon (Cucumis melo L.) seeds as potential source for oils. Foods 2020, 9, 1341. [Google Scholar] [CrossRef]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Nutritional composition and health benefits of various botanical types of melon (Cucumis melo L.). Plants 2021, 10, 1755. [Google Scholar] [CrossRef]
- Ahrolovich, R.N.; Madiyarovich, S.S.; Urinbaevana, M.H. Melon and its environmental characteristics. J. Crit. Rev. 2020, 7, 480–490. [Google Scholar] [CrossRef]
- Buczkowska, H.; Nurzyńska-Wierdak, R. Fruiting of melon (Cucumis melo L.) grown organically on mulched soil. Acta Sci. Pol. Hortorum Cultus 2020, 19, 121–131. [Google Scholar] [CrossRef]
- Insanu, M.; Rizaldy, D.; Silviani, V.; Fidrianny, I. Chemical compounds and pharmacological activities of Cucumis genus. Biointerface Res. Appl. Chem. 2022, 12, 1324–1334. [Google Scholar] [CrossRef]
- Zulfikar, M.; Widya, F.S.; Wibowo, W.A.; Daryono, B.S.; Widiyanto, S. Antioxidant activity of melon fruit (Cucumis melo L.‘GMP’) ethanolic extract. AIP Conf. Proc. 2020, 2260, 040029. [Google Scholar] [CrossRef]
- Rolbiecki, R.; Rolbiecki, S.; Figas, A.; Jagosz, B.; Wichrowska, D.; Ptach, W.; Prus, P.; Sadan, H.A.; Ferenc, P.-F.; Stachowski, P.; et al. Effect of drip fertigation with nitrogen on yield and nutritive value of melon cultivated on a very light soil. Agronomy 2021, 11, 934. [Google Scholar] [CrossRef]
- Neocleous, D.; Ntatsi, G.; Savvas, D. Physiological, nutritional and growth responses of melon (Cucumis melo L.) to a gradual salinity built-up in recirculating nutrient solution. J. Plant Nutr. 2017, 40, 2168–2180. [Google Scholar] [CrossRef]
- Dogan, E.; Kirnak, H.; Berekatoglu, K.; Bilgel, L.; Surucu, A. Water stress imposed on muskmelon (Cucumis melo L.) with subsurface and surface drip irrigation systems under semi-arid climatic conditions. Irrig. Sci. 2008, 26, 131–138. [Google Scholar] [CrossRef]
- Al-Mefleh, N.K.; Samarah, N.; Zaitoun, S.; Al-Ghzawi, A. Effect of irrigation levels on fruit characteristics, total fruit yield and water use efficiency of melon under drip irrigation system. J. Food Agric. Environ. 2012, 10, 540–545. [Google Scholar]
- Nut, N.; Phou, K.; Mihara, M.; Nuth, S.; Sor, S. Effects of Drip Irrigation Frequency on Growth and Yield of Melon (Cucumis melo L.) Under Net-House’s Conditions. Int. J. Environ. Rural Dev. 2019, 10, 146–152. [Google Scholar]
- Ibrahim, E.A. Response of some Egyptian sweet melon (Cucumis melo var. aegyptiacus L.) cultivars to water stress conditions. J. Appl. Hortic. 2012, 14, 67–70. [Google Scholar] [CrossRef]
- Barzegar, T.; Lotfi, H.; Rabiei, V.; Ghahremani, Z.; Nikbakht, J. Effect of water-deficit stress on fruit yield, antioxidant activity, and some physiological traits of four Iranian melon genotypes. Iran. J. Hortic. Sci. 2017, 48, 13–25. [Google Scholar] [CrossRef]
- Akhoundnejad, Y.; Daşgan, H.Y. Effect of different irrigation levels on physiological performance of some drought tolerant melon (Cucumis melo L.) genotypes. Appl. Ecol. Environ. Res. 2019, 17, 9997–10012. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; Martínez-Sánchez, G.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Blanca, J.; Yenush, L.; Mulet, J.M. Distinctive traits for drought and salt stress tolerance in melon (Cucumis melo L.). Front. Plant Sci. 2021, 12, 2471. [Google Scholar] [CrossRef]
- Pandey, S.; Ansari, W.A.; Jha, A.; Bhatt, K.V.; Singh, B. Evaluation of melons and indigenous Cucumis spp. genotypes for drought tolerance. Acta Hortic. 2011, 979, 335–339. [Google Scholar] [CrossRef]
- Kıran, S.; Furtana, G.B.; Talhouni, M.; Ellialtıoğlu, Ş.Ş. Drought stress mitigation with humic acid in two Cucumis melo L. genotypes differ in their drought tolerance. Bragantia 2019, 78, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Long, R.L.; Walsh, K.B.; Midmore, D.J.; Rogers, G. Irrigation scheduling to increase muskmelon fruit biomass and soluble solids concentration. Hortscience 2006, 41, 367–369. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 306. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, S.; Hassandokht, M.R.; Mirsoleimani, A.; Mousavi, A. Effect of palm leaf biochar on melon plants (Cucumis melo L.) under drought stress conditions. Adv. Hortic. Sci. 2019, 33, 593–604. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P.; Alyemeni, M.N.; Alsahli, A.A.; Ahmad, P. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi J. Biol. Sci. 2021, 28, 1465–1476. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Noor, H.M.; Ahmad, H.; Sayuti, Z. Effect of mycorrhiza, fertilizers and planting media on rock melon (Cucumis melo Linn Cv. Glamour) growth using the canopytechture structure. Int. J. Appl. Agric. Sci. 2019, 5, 14. [Google Scholar] [CrossRef]
- Cakmakci, O.; Cakmakci, T.; Durak, E.D.; Demir, S.; Sensoy, S. Effects of arbuscular mycorrhizal fungi in melon (Cucumis melo L.) seedling under deficit irrigation. Fresenius Environ. Bull. 2017, 26, 7513–7520. [Google Scholar]
- Dere, S.; Coban, A.; Akhoundnejad, Y.; Ozsoy, S.; Dasgan, H.Y. Use of mycorrhiza to reduce mineral fertilizers in soilless melon (Cucumis melo L.) cultivation. Not. Bot. Horti. Agrobot. Cluj-Napoca 2019, 47, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Vetrano, F.; Torta, L.; Esposito, A.; Moncada, A. Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy 2023, 13, 440. [Google Scholar] [CrossRef]
- Franczuk, J.; Rosa, R.; Kosterna-Kelle, E.; Zaniewicz-Bajkowska, A.; Panasz, M. The effect of transplanting date and covering on the growth and development of melon (Cucumis melo L.). Acta Agrobot. 2017, 70, 1699. [Google Scholar] [CrossRef] [Green Version]
- Majkowska-Gadomska, J. Mineral content of melon fruit (Cucumis melo L.). J. Elementol. 2009, 14, 717–727. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Michałek, W. Effect of mycorrhiza inoculation of pepper seedlings (Capsicum annuum L.) on the growth and protection against Fusarium oxysporum infection. Acta Sci. Pol. Hortorum Cultus 2019, 18, 161–169. [Google Scholar] [CrossRef]
- PN-90/A-75101.02; Przetwory Owocowe i Warzywne. Przygotowanie Próbek i Metody Badań Fizykochemicznych. Oznaczanie Zawartości Ekstraktu Ogólnego. (Fruit and Vegetable Preserves. Preparation of Samples and Methods of Physicochemical Tests. Determination of the Total Extract Content). Polski Komitet Normalizacyjny: Warsaw, Poland, 2002.
- Najda, A.; Klimek, K.; Buczkowska, H.; Balant, S.; Wrzesinska-Jedrusiak, E. Effect of ozonated water on the content of bioactive phenolic compounds and shelf-life of fresh coriander (Coriandrum sativum L.). Przem. Chem. 2019, 98, 1286–1289. [Google Scholar] [CrossRef]
- Lees, R. Food Analysis: Analytical and Quality Control Methods for the Manufacturer and Buyer; Leonard Hill Books: London, UK, 1975; pp. 145–146. [Google Scholar]
- Mínguez-Mosquera, M.I.; Jaren-Galán, M.; Garrido-Fernández, J. Color quality in paprika. J. Agric. Food Chem. 1992, 40, 2384–2388. [Google Scholar] [CrossRef]
- Horváth, L.; Gyulai, G.; Szabó, Z.; Lágler, R.; Tóth, Z.; Heszky, L. Morphological diversity of current melons (Cucumis melo) compared to a medieval type. Acta Agrar. Debr. 2007, 27, 84–90. [Google Scholar] [CrossRef]
- Rad, M.R.N.; Ghasemi, M.M.; Koohpayegani, J.A. Evaluation of melon (Cucumis melo. L) genotypes aiming effective selection of parents for breeding directed at high yield under drought stress condition. J. Hortic. Res. 2017, 25, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, N.; Kaya, N.; Pınar, H.; Hancı, F.; Uzun, A. Detailed morphological and molecular characterizations of melon (Cucumis melo L.) accessions collected from Northern Cyprus and Turkey. Hortic. Sci. Technol. 2021, 39, 471–481. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Liang, R.; Su, Y.; Qin, X.; Gao, Z.; Fu, Z.; Qiu, H.; Lin, X.; Zhu, J. Comparative transcriptomic analysis of two Cucumis melo var. saccharinus germplasms differing in fruit physical and chemical characteristics. BMC Plant Biol. 2022, 22, 193. [Google Scholar] [CrossRef]
- Yu, G.; Zhu, G.; Zheng, X. A hami melon flavor creation. Food Sci. Technol. Camp. 2022, 42, e95221. [Google Scholar] [CrossRef]
- Albuquerque, B.; Lidon, F.C.; Barreiro, M.G. A case study on the flavor properties of melon (Cucumis melo L.) cultivars. Fruits 2006, 61, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Bouzo, C.A.; Céccoli, G.; Muñoz, F. Effect of potassium and calcium upon the yield and fruit quality of Cucumis melo. Agriscientia 2018, 35, 25–33. [Google Scholar] [CrossRef]
- de Melo, A.S.; Dias, V.G.; Dutra, W.F.; Dutra, A.F.; Sá, F.V.D.S.; Brito, M.E.B.; Viégas, P.R.A. Physiology and yield of Piel de sapo melon (Cucumis melo L.) under water deficit in semi-arid region, Brasil. Biosci. J. Uberlândia 2020, 36, 1251–1260. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Gallardo, M.; Thompson, R.B. Water and fertilization management of vegetables: State of art and future challenges. Eur. J. Hortic. Sci. 2018, 83, 306–318. [Google Scholar] [CrossRef]
- Abraham-Juárez, M.; Espitia-Vázquez, I.; Guzmán-Mendoza, R.; Olalde-Portugal, V.; Ruiz-Aguilar, G.M.; García-Hernández, J.L.; Herrera-Isidrón, L.; Núñez-Palenius, H.G. Development, yield, and quality of melon fruit (Cucumis melo L.) inoculated with Mexican native strains of Bacillus subtilis (Ehrenberg). Agrociencia 2018, 52, 91–102. [Google Scholar]
- Gómez-Bellot, M.J.; Lorente, B.; Sánchez-Blanco, M.J.; Ortuño, M.F.; Nortes, P.A.; Alarcón, J.J. Influence of mixed substrate and arbuscular mycorrhizal fungi on photosynthetic efficiency, nutrient and water status and yield in tomato plants irrigated with saline reclaimed waters. Water 2020, 12, 438. [Google Scholar] [CrossRef] [Green Version]
| Year | Mineral Components (mg·dm−3) | pHH2O | Salinity (mg KCL·dm−3) | ||||
|---|---|---|---|---|---|---|---|
| N-NO3 | P | K | Ca | Mg | |||
| 2018 | 37 | 75 | 128 | 1480 | 105 | 6.5 | 0.17 |
| 2019 | 45 | 90 | 145 | 1250 | 90 | 6.4 | 0.23 |
| 2020 | 25 | 87 | 160 | 1620 | 110 | 6.7 | 0.25 |
| Year | Sowing Seeds | Thinning Seedlings | Soil Mulching | Planting Seedling | First Harvest | Last Harvest |
|---|---|---|---|---|---|---|
| 2018 | 14.05 | 29.05 | 8.06 | 14.06 | 7.08 | 1.09 |
| 2019 | 15.05 | 27.05 | 6.06 | 13.06 | 9.08 | 4.09 |
| 2020 | 12.05 | 27.05 | 7.06 | 12.06 | 10.08 | 2.09 |
| Year | Temperature (°C) | Precipitation (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| June | July | August | September | Mean | June | July | August | September | ∑ | |
| 2018 | 18.8 | 20.5 | 20.8 | 15.5 | 18.9 | 65 | 125 | 72 | 70 | 332 |
| 2019 | 21.5 | 19.4 | 20.3 | 15.5 | 19.2 | 37 | 38 | 102 | 68 | 245 |
| 2020 | 23.1 | 19.2 | 20.3 | 15.3 | 19.5 | 168 | 29 | 45 | 172 | 414 |
| 1951–2010 | 16.3 | 18.0 | 17.2 | 12.6 | 16.0 | 66 | 82 | 71 | 54 | 273 |
| Cultivar | Treatment | Total Fruit Number (TF) (pcs·m−2) | Marketable Fruit Number (MF) (pcs·m−2) | MF in TF (%) | Total Yield (TY) (kg·m−2) | Marketable Yield (MY) (kg·m−2) | MY in TY (%) |
|---|---|---|---|---|---|---|---|
| Melba | AMF and IR * | 5.2 ± 0.6 a | 4.6 ± 0.5 a | 88.5 | 3.55 ± 0.54 bcd | 3.01 ± 0.39 bc | 84.8 |
| AMF and non-IR | 5.0 ± 0.8 ab | 4.4 ± 0.8 ab | 88.0 | 3.23 ± 0.67 cde | 2.67 ± 0.5 ab | 82.7 | |
| non-AMF and IR | 4.9 ± 0.8 ab | 4.4 ± 0.7 ab | 89.8 | 3.06 ± 0.56 de | 2.5 ± 0.47 cde | 81.7 | |
| non-AMF and non-IR | 4.6 ± 0.7 bc | 3.9 ± 0.7 bc | 84.5 | 2.71 ± 0.47 e | 2.12 ± 0.40 e | 78.2 | |
| Mean | 4.9 ± 0.7 A | 4.3 ± 0.7 A | 88.7 | 3.14 ± 0.63 A | 2.58 ± 0.54 B | 82.2 | |
| Emir F1 | AMF and IR * | 4.6 ± 0.7 abc | 3.9 ± 0.4 bc | 84.8 | 4.29 ± 0.73 a | 3.75 ± 0.53 a | 87.4 |
| AMF and non-IR | 4.2 ± 0.6 bcde | 3.5 ± 0.5 cde | 83.3 | 4.16 ± 0.58 ab | 3.41 ± 0.42 ab | 82.0 | |
| non-AMF and IR | 3.8 ± 0.7 cde | 3.0 ± 0.8 def | 78.9 | 3.64 ± 0.51 abcd | 2.87 ± 0.64 bcd | 78.8 | |
| non-AMF and non-IR | 3.4 ± 0.8 d | 2.7 ± 0.6 f | 79.4 | 3.12 ± 0.48 | 2.50 ± 0.40 cde | 80.1 | |
| Mean | 4.0 ± 0.9 B | 3.3 ± 0.7 B | 82.5 | 3.80 ± 0.85 B | 3.13 ± 0.74 A | 82.4 | |
| Seledyn F1 | AMF and IR * | 4.4 ± 0.6 bcd | 3.8 ± 0.4 bc | 86.4 | 4.30 ± 0.32 a | 3.72 ± 0.30 a | 86.5 |
| AMF and non-IR | 4.1 ± 0.3 cde | 3.3 ± 0.3 cdef | 80.5 | 4.02 ± 0.32 ab | 3.29 ± 0.37 ab | 81.8 | |
| non-AMF and IR | 3.9 ± 0.4 cde | 3.2 ± 0.5 cdef | 82.0 | 3.69 ± 0.40 abcd | 3.14 ± 0.38 bc | 85.1 | |
| non-AMF and non-IR | 3.4 ± 0.4 e | 2.8 ± 0.4 ef | 82.3 | 3.27 ± 0.22 cde | 2.55 ± 0.25 cde | 78.0 | |
| Mean | 4.0 ± 0.6 B | 3.3 ± 0.5 B | 82.5 | 3.82 ± 0.50 B | 3.18 ± 0.53 A | 83.4 | |
| Oliwin F1 | AMF and IR * | 4.3 ± 0.6 bcd | 3.6 ± 0.6 bc | 83.7 | 3.92 ± 0.57 abc | 3.30 ± 0.50 ab | 84.2 |
| AMF and non-IR | 4.0 ± 0.5 cde | 3.2 ± 0.5 cdef | 80.0 | 3.49 ± 0.42 bcd | 2.92 ± 0.42 bcd | 83.7 | |
| non-AMF and IR | 3.7 ± 0.4 de | 3.0 ± 0.3 def | 81.1 | 3.18 ± 0.24 de | 2.45 ± 0.25 cde | 77.0 | |
| non-AMF and non-IR | 3.7 ± 0.5 de | 2.9 ± 0.5 def | 78.4 | 3.05 ± 0.26 | 2.37 ± 0.29 de | 77.7 | |
| Mean | 3.9 ± 0.5 B | 3.2 ± 0.5 B | 82.1 | 3.41 ± 0.51 A | 2.76 ± 0.53 B | 80.9 | |
| Mean | AMF and IR * | 4.6 ± 0.7 A | 4.0 ± 0.6 A | 87.0 | 4.02 ± 0.62 A | 3.45 ± 0.53 A | 85.8 |
| AMF and non-IR | 4.3 ± 0.7 B | 3.6 ± 0.7 B | 83.7 | 3.72 ± 0.63 B | 3.07 ± 0.50 B | 82.5 | |
| non-AMF and IR | 4.1 ± 0.8 C | 3.4 ± 0.9 B | 82.9 | 3.39 ± 0.66 C | 2.74 ± 0.58 C | 80.8 | |
| non-AMF and non-IR | 3.8 ± 0.8 C | 3.1 ± 0.7 C | 81.6 | 3.04 ± 0.42 D | 2.38 ± 0.37 D | 78.3 | |
| Mean | 4.2 ± 0.8 | 3.5 ± 0.8 | 83.3 | 3.54 ± 0.69 | 2.91 ± 0.64 | 82.2 | |
| Mean | 2018 | 4.0 ± 0.9 B | 3.4 ± 0.8 A | 85.0 | 3.44 ± 0.80 A | 2.86 ± 0.71 A | 83.1 |
| 2019 | 4.3 ± 0.8 A | 3.5 ± 0.8 A | 81.4 | 3.64 ± 0.69 A | 2.98 ± 0.65 B | 81.9 | |
| 2020 | 4.3 ± 0.8 A | 3.5 ± 0.8 A | 81.4 | 3.54 ± 0.60 A | 2.89 ± 0.54 A | 81.6 |
| Cultivar | Treatment | Fruit Weight (FW) (g) | Flesh Weight (FLW) (g) | FLW in FW (%) | Skin Thickness (mm) | Flesh Thickness (cm) |
|---|---|---|---|---|---|---|
| Melba | AMF and IR * | 655 ± 65 d | 585 ± 55 d | 89.3 | 4.2 ±0.1 a | 2.6 ± 0.1 gh |
| AMF and non-IR | 613 ± 44 d | 543 ± 39 de | 88.6 | 4.0 ± 0.2 ab | 2.5 ± 0.1 hi | |
| non-AMF and IR | 567 ± 42 d | 499 ± 37 de | 88.0 | 4.2 ± 0.1 abc | 2.5 ± 0.1 hi | |
| non-AMF and non-IR | 555 ± 68 d | 488 ± 61 d | 87.9 | 4.1 ± 0.3 bcde | 2.4 ± 0.1 i | |
| Mean | 597 ± 67 C | 529 ± 61 C | 88.6 | 4.1 ± 0.2 A | 2.5 ± 0.1 D | |
| Emir F1 | AMF and IR * | 970 ± 74 a | 836 ± 59 a | 86.2 | 4.0 ± 0.2 abcde | 3.7 ± 0.1 a |
| AMF and non-IR | 982 ± 61 a | 848 ± 54 a | 86.4 | 3.9 ± 0.1 cde | 3.6 ± 0.2 ab | |
| non-AMF and IR | 959 ± 69 a | 828 ± 60 a | 86.3 | 4.0 ± 0.2 cde | 3.5 ± 0.1 bc | |
| non-AMF and non-IR | 930 ± 62 ab | 803 ± 55 ab | 86.3 | 3.9 ± 0.2 abcd | 3.4 ± 0.1 cde | |
| Mean | 960 ± 68 A | 829 ± 58 A | 86.4 | 4.0 ± 0.2 B | 3.6 ± 0.2 A | |
| Seledyn F1 | AMF and IR * | 1011 ± 131 a | 876 ± 114 | 86.6 | 3.9 ± 0.2 de | 3.6 ± 0.1 bc |
| AMF and non-IR | 1010 ± 122 a | 878 ± 106 a | 86.9 | 3.8 ± 0.2 de | 3.5 ± 0.2 bcd | |
| non-AMF and IR | 994 ± 83 a | 859 ± 70 a | 86.4 | 4.1 ± 0.2 abcde | 3.4 ± 0.1 cde | |
| non-AMF and non-IR | 939 ± 123 ab | 819 ± 111 a | 87.2 | 4.0 ± 0.1 abcde | 3.4 ± 0.1 cde | |
| Mean | 988 ± 115 A | 858 ± 101 A | 86.8 | 3.9 ± 0.2 B | 3.5 ± 0.1 B | |
| Oliwin F1 | AMF and IR * | 924 ± 49 abc | 800 ± 43 abc | 86.6 | 3.8 ± 0.2 e | 3.4 ± 0.1 de |
| AMF and non-IR | 910 ± 97 abc | 788 ± 85 abc | 86.6 | 3.9 ± 0.2 de | 3.3 ± 0.2 e | |
| non-AMF and IR | 840 ± 112 bc | 726 ± 98 bc | 86.4 | 3.9 ± 0.1 bcde | 3.1± 0.2 f | |
| non-AMF and non-IR | 822 ± 94 c | 711 ± 83 c | 86.5 | 3.9 ± 0.1 bcde | 2.7 ±0.3 g | |
| Mean | 874 ± 99 B | 756 ± 86 B | 86.5 | 3.9 ± 0.2 B | 3.1 ± 0.3 C | |
| Mean | AMF and IR * | 890 ± 164 A | 744 ± 134 A | 87.0 | 4.0 ± 0.2 A | 3.4 ± 0.5 A |
| AMF and non-IR | 879 ± 180 AB | 764152 A | 86.9 | 3.9 ± 0.2 A | 3.3 ± 0.5 A | |
| non-AMF and IR | 840 ± 187 BC | 728 ± 158 B | 86.7 | 4.0 ± 0.2 A | 3.2 ± 0.4 B | |
| non-AMF and non-IR | 812 ± 180 C | 705 ± 155 B | 86.8 | 4.0 ± 0.2 A | 3.0 ± 0.5 C | |
| Mean | 855 ± 179 | 743 ± 151 | 86.9 | 4.0 ± 0.2 | 3.2 ± 0.5 | |
| Mean | 2018 | 855 ± 167 A | 744 ± 143 AB | 87.0 | 4.0 ± 0.2 A | 3.1 ± 0.5 A |
| 2019 | 870 ± 209 A | 757 ± 176 A | 87.0 | 4.0 ± 0.3 A | 3.2 ± 0.4 B | |
| 2020 | 840 ± 159 A | 727 ± 132 B | 86.5 | 3.9 ± 0.2 A | 3.2 ± 0.5 B |
| Cultivar | Treatment | Dry Matter (%) | Soluble Solids (%) | L-Ascorbic Acid (mg · 100 g−1 FM) | Total Sugars (% FM) | Reducing Sugars (% FM) | Carotenoids (µg · 100 g−1 FM) |
|---|---|---|---|---|---|---|---|
| Melba | AMF and IR * | 4.82 ± 0.45 ghi | 7.05 ± 0.49 cd | 15.13 ± 0.67 ab | 5.55 ± 0.81 d | 3.07 ± 0.49 cd | 987.2 ± 215.7 ab |
| AMF and non-IR | 4.88 ± 0.36 ghi | 6.70 ± 0.45 ef | 15.32 ± 0.64 ab | 5.49 ± 0.79 d | 3.01 ± 0.31 d | 1016.8 ± 271.5 a | |
| non-AMF and IR | 4.72 ± 0.48 hi | 6.67 ± 0.49 ef | 14.75 ± 0.59 bc | 5.06 ± 0.87 ef | 32.61 ± 0.46 efg | 907.7 ± 199.3 c | |
| non-AMF and non-IR | 4.80 ± 0.48 hi | 6.85 ± 0.51 de | 14.52 ± 0.68 bc | 5.23 ± 0.74 e | 2.64 ± 0.36 ef | 887.3 ± 176.7 c | |
| Mean | 4.81 ± 0.42 D | 6.82 ± 0.48 B | 14.93 ± 0.57 B | 5.33 ± 0.79 C | 2.82 ± 0.39 B | 949.7 ± 211.1 A | |
| Emir F1 | AMF and IR * | 6.04 ± 0.80 b | 8.28 ± 0.75 a | 14.27 ± 0.35 cd | 6.92 ± 0.51 a | 3.53 ± 0.74 a | 648.2 ± 116.1 e |
| AMF and non-IR | 6.06 ± 0.52 b | 7.22 ± 0.47 bc | 13.54 ± 0.75 de | 6.87 ± 0.42 a | 3.38 ± 0.75 ab | 611.0 ± 131.8 f | |
| non-AMF and IR | 5.84 ± 0.73 cd | 7.47 ± 0.85 b | 13.63 ± 0.79 d | 6.41 ± 0.23 bc | 3.26 ± 0.76 b | 593.2 ± 130.3 fg | |
| non-AMF and non-IR | 6.35 ± 0.76 a | 8.18 ± 0.63 a | 13.42 ± 0.75 de | 6.37 ± 0.39 c | 3.22 ± 0.78 bc | 583.5 ± 132.2 fg | |
| Mean | 6.07 ± 0.69 A | 7.79 ± 0.63 A | 13.72 ± 0.73 C | 6.64 ± 0.33A | 3.35 ± 0.71 A | 609.0 ± 121.8 C | |
| Seledyn F1 | AMF and IR * | 5.53 ± 0.27 ef | 6.05 ± 0.49 b | 13.47 ± 0.67 de | 6.57 ± 0.13 b | 2.74 ± 0.19 e | 583.2 ± 81.4 fg |
| AMF and non-IR | 5.84 ± 0.43 c | 6.72 ± 0.37 ef | 13.54 ± 0.59 de | 6.46 ± 0.14 bc | 2.54 ± 0.16 fgh | 570.2 ± 68.6 g | |
| non-AMF and IR | 5.37 ± 0.39 f | 6.12 ± 0.45 h | 13.43 ± 0.69 de | 6.34 ± 0.17 c | 2.43 ± 0.17 ghi | 450.5 ± 68.0 h | |
| non-AMF and non-IR | 5.63 ± 0.48 de | 6.48 ± 0.48 fg | 12.73 ± 0.35 e | 6.35 ± 0.19 c | 2.36 ± 0.14 hi | 449.8 ± 56.8 h | |
| Mean | 5.59 ± 0.42 B | 6.34 ± 0.68 C | 13.25 ± 0.42 D | 6.43 ± 0.15 B | 2.52 ± 0.19 C | 513.4 ± 82.0 D | |
| Oliwin | AMF and IR * | 4.92 ± 0.37 gh | 6.31 ± 0.38 gh | 15.85 ± 0.45 a | 4.89 ± 0.11 fg | 2.28 ± 0.26 ij | 987.5 ± 289.9 ab |
| AMF and non-IR | 4.90 ± 0.53 gh | 6.25 ± 0.25 gh | 15.69 ± 0.48 a | 4.78 ± 0.19 gh | 2.13 ± 0.198 jk | 957.3 ± 257.9 b | |
| non-AMF and IR | 4.69 ± 0.54 i | 6.08 ± 0.29 h | 16.62 ± 0.47 a | 4.61 ± 0.13 hi | 2.10 ± 0.25 jk | 811.8 ± 261.6 d | |
| non-AMF and non-IR | 5.01 ± 0.44 g | 6.06 ± 0.34 h | 15.26 ± 0.42 ab | 4.53 ± 0.12 i | 2.07 ± 0.17 j | 790.8 ± 272.6 d | |
| Mean | 4.88 ± 0.46 C | 6.18 ± 0.65 D | 15.61 ± 0.44 A | 4.70 ± 0.18 D | 2.15 ± 0.22 D | 886.8 ± 285.8 B | |
| Mean | AMF and IR * | 5.33 ± 0.50 B | 6.92 ± 0.42 A | 14.68 ± 0.54 A | 5.98 ± 092 A | 2.91 ± 0.49 A | 801.5 ± 290.1 A |
| AMF and non-IR | 5.42 ± 0.54 A | 6.72 ± 0.66 B | 14.52 ± 0.53 AB | 5.90 ± 0.92 B | 2.77 ± 0.35 B | 788.8 ± 200.6 A | |
| non-AMF and IR | 5.15 ± 0.33 C | 6.59 ± 0.29 C | 14.36 ± 0.57 B | 5.60 ± 0.91 C | 2.60 ± 0.39 C | 690.8 ± 146.7 B | |
| non-AMF and non-IR | 5.45 ± 0.41 A | 6.89 ± 0.38 A | 13.98 ± 0.52 C | 5.62 ± 0.88 C | 2.57 ± 0.33 C | 677.8 ± 139.7 B | |
| Mean | 5.34 ± 0.49 | 6.78 ± 0.58 | 14.39 ± 0.53 | 5.78 ± 0.91 | 2.71 ± 0.81 | 739.7 ± 272.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczkowska, H.; Sałata, A.; Nurzyńska-Wierdak, R. Melon (Cucumis melo L.) Fruit Yield under Irrigation and Mycorrhiza Conditions. Agronomy 2023, 13, 1559. https://doi.org/10.3390/agronomy13061559
Buczkowska H, Sałata A, Nurzyńska-Wierdak R. Melon (Cucumis melo L.) Fruit Yield under Irrigation and Mycorrhiza Conditions. Agronomy. 2023; 13(6):1559. https://doi.org/10.3390/agronomy13061559
Chicago/Turabian StyleBuczkowska, Halina, Andrzej Sałata, and Renata Nurzyńska-Wierdak. 2023. "Melon (Cucumis melo L.) Fruit Yield under Irrigation and Mycorrhiza Conditions" Agronomy 13, no. 6: 1559. https://doi.org/10.3390/agronomy13061559