- *Corresponding Author:
- V. B. Tatipamula
Center for Molecular Biology, College of Medicine and Pharmacy, Duy Tan University, Danang 550000, Vietnam
E-mail: vinaybharadwajtatipamula@duytan.edu.vn
| Date of Received | 17 June 2022 |
| Date of Revision | 28 February 2023 |
| Date of Acceptance | 20 June 2023 |
| Indian J Pharm Sci 2023;85(3):799-805 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the present study is to evaluate the pharmacological actions of earlier reported metabolites (1-5), and semi-synthetic analogs (4a-e) from acetone extract of Ramalina leiodea. The Acetone extract of Ramalina leiodea were screened for antioxidant, anti-inflammatory, anticancer, and acute toxicity studies. The antioxidant results exhibited that compound 4e showed effective inhibitory ability against ferric ions, 2,2-diphenyl-1-picrylhydrazyl and superoxide free radicals with 50 % inhibition value of 28.50, 27.0, and 25.0 μg/ml, respectively. Also, among all the samples, 4e revealed good inhibition of albumin denaturation of 105 μg/ml (IC50 value). Additionally, the median lethal dosage of acetone extract of Ramalina leiodea was observed to be above 2000 mg/Kg body weight. Also, the in vivo antiinflammatory studies of acetone extract of Ramalina leiodea exhibited a noticeable reduction of edema in rat paw at both low and high dosage when compared to indomethacin. From the sulforhodamine B assay, it is concluded that 2 and 4b displayed a noteworthy degree of specificity against tested series of cell lines. On the other hand, all tested samples displayed a low degree of specificity against normal cells. To conclude, Ramalina leiodea could be the best source for bioactive agents for the management of oxidative stress, inflammation, and cancer.
Keywords
Derivatization, antioxidant, protein denaturation, anti-inflammatory, anticancer
Ramalina genus has about 246 species distributed around the world, of which only 118 species were investigated for their chemical and biological studies[1]. A diversity of secondary metabolites were isolated which include usnic acid derivatives[2], depsides[3], depsidones[3,4], fatty acids[5,6], sterols[5-7] and monocyclic aromatic compounds[7-9]. Moreover, the biological screening of this genus results in identification of antibiotic[9], antimutagenic[10], anti- HIV[11,12], enzyme inhibitory[4,13-15], antioxidant[16], anti-inflammatory[17], anticancer[18], and antimicrobial[19] activities.
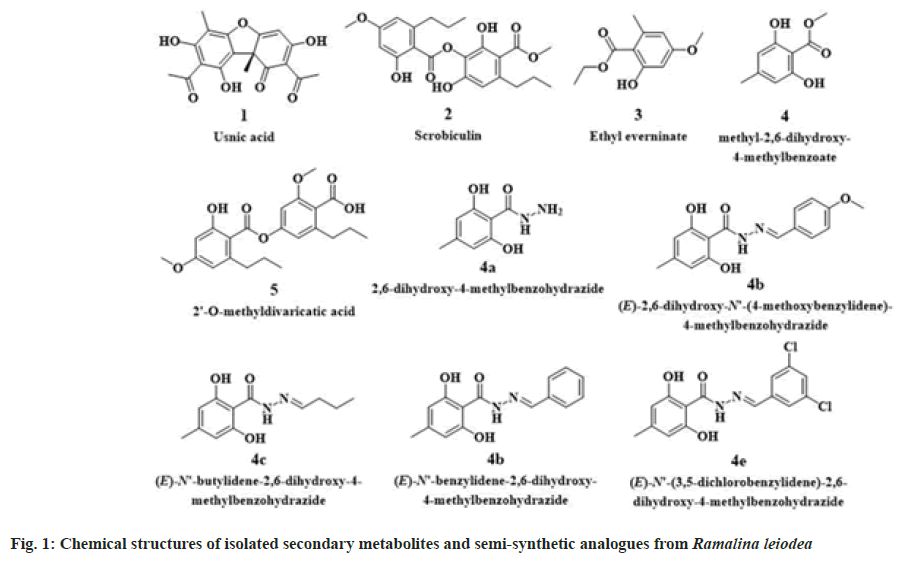
Ramalina leiodea (Nyl.) Nyl. (R. leiodea)(family Ramalinaceae) is a corticolous fruticose lichen, belongs to the genus Ramalina[1]. Previously, biological activities include antibacterial[19], antifungal[19], anti-tubercular[19], tyrosinase inhibitory[13,14] and anti-inflammatory[1,17] activities were reported from R. leiodea. Also, a depside olivetoric acid was isolated and reported from lichen R. leiodea[1]. Earlier our group[19], in the course of a chemical examination of Acetone Extract of lichen R. leiodea (AE), obtained five known metabolites (1-5) (fig. 1). Of which highly yielded compound 4 was subjected to synthetic methods using hydrazine hydrate yielded novel benzohydrazides (4a-e) (fig. 1), which were claimed to have potent antimicrobial and anti-tubercular activities[19]. An extension of our investigation to detect bioactive secondary metabolites from R. leiodea. The existing study was implemented to determine the biological profile of chemical constituents, as well as, semi-synthetic derivatives of R. leiodea.
Materials and Methods
Materials:
The materials are extracted, isolated, and synthesized according to the protocols of Tatipamula and Vedula, 2019[19].
Antioxidant activity:
1,1-diphenyl-2-picrylhydrazyl (DPPH) assay: By employing DPPH assay, the secondary metabolites (1-5), analogs (4a-e), and AE were exposed to antioxidant activity in triplicate[21]. To 0.004 % DPPH, known concentrations of the test samples are added and incubated at 37° for 30 min and then measured absorbance at 517 nm against the blank.
Ferric ion (Fe3+) reducing power assay: By employing Fe3+ assay, the secondary metabolites (1-5), analogs (4a-e), and AE were exposed to antioxidant activity in triplicate[22]. To prepared 1 % potassium ferricyanide solution with phosphate buffer, added know concentrations of the test sample, 0.1 % of ferric chloride and 10 % trichloroacetic acid, and incubated at 37° for 20 min, and detected absorbance at 700 nm against the blank.
Superoxide radical scavenging assay: By employing superoxide radicals, the secondary metabolites (1-5), analogs (4a-e), and AE were exposed to antioxidant activity in triplicate[24]. To prepared Nicotinamide Adenine Dinucleotide, Phenazine Methosulphate (PMS) and NBT (Nitroblue Tetrazolium Chloride) added know concentrations of the test sample and incubated at 37° for 30 min and noted absorbance at 562 nm against the blank.
In vitro anti-inflammatory activity:
Employing the protein denaturation method[25], the secondary metabolites (1-5), analogs (4a-e), and AE were determined for their in vitro anti- inflammatory activity in triplicate. Albumin protein from bovine serum was used as a protein. By using sodium phosphate buffer, the protein was solubilized. To the 0.2 ml of the prepared protein sample, added know concentrations of the test sample, and finally made up the volume to 5 ml with the above buffer. All the prepared samples are incubated at 37° for 20 min, then boiled at 95° for 20 and chilled to 25°, noted absorbance at 562 nm against the blank.
Animals:
As per the OECD regulations, the present experiments are designed and registered with number 12/IAEC/SVCP/2021-22. For the present study, albino rats of both sexes weigh 180-200 g are used.
Acute toxicity studies:
To healthy male albino rats (n=5), dosed orally with AE at 2000 mg/kg body weight (b.w) and observed for 24 h[21]. Based on the mortality, the median Lethal Dose (LD50) of AE was estimated.
In vivo anti-inflammatory activity:
Employing formalin-induced hind albino rat (either sex; n=6) paw edema assay[26], AE was subject to in vivo anti-inflammatory activity by plethysmographic measurement. Set I is for control (treated intraperitoneally with 0.5 % Carboxymethylcellulose). Set II for standard drug, indomethacin (administered intraperitoneally with 100 mg/Kg b.w). Set III and IV aided for AE at a low dose (intraperitoneally 100 mg/Kg b.w) and high dose (intraperitoneally 200 mg/Kg b.w), respectively. 0.1 ml of formalin (1 % w/v) was injected to plantar area of rat left paw, after 30 min of administration of test samples to albino rats, and measured paw volume at 2 and 4 h.
Percentage reduction=Volume of paw oedema in controlled animals-Volume of paw oedema in treated animals/Volume of paw oedema in controlled animals×100
Anticancer activity:
Utilizing the SRB assay[27], the secondary metabolites (1-5), analogs (4a-e), and AE were implemented for their in vitro anticancer activity in triplicate (n=3) using three cancer cells viz. Breast (MCF-7), Cervical (HeLa), Colon (DLD- 1), Head and Neck (FADU), Lung (A549), and one normal cell line; Normal Human Mammary Epithelial (NHME). All the cells are purchased in good order from National Centre for Cell Science, Pune and preserved according to the procedures of Haritha et al.[22]. To 190 µl suspension of cancer cells added test samples (dissolved in dimethyl sulfoxide) and incubated at 37° for 3 h in the presence of 5 % CO2. Later, added cold TCA (100 µl) and incubate at 4° for 1 h, then washed and dried to room temperature. To it added 0.057 % Sulforhodamine B solution (100 µl) and incubated for 30 min. Finally, the 96-well plate is stained and detect the optical density at 510 nm.
Percentage inhibition=100-[Absorbance of sample/ Absorbance of control×100
Statistical analysis:
The in vitro values are expressed as mean±SEM values of three independent tests. While in vivo, values are expressed as mean±SEM values of six independent tests compared with one-way ANOVA followed by Dunnett’s test, where p<0.05 found to be statistically significant among the experimental groups.
Results and Discussion
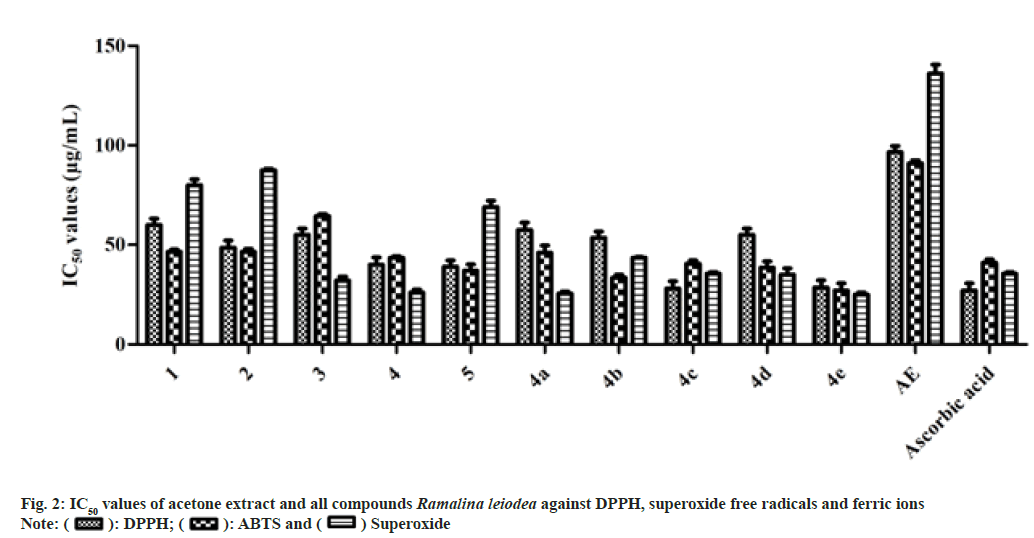
The antioxidant activity results are illustrated in fig. 2, from the data it is determined that the 50 % inhibition of DPPH free radicals of 1, 2, 3, 4, 5, 4a, 4b, 4c, 4d, 4e and AE were determined to be 60.0, 48.50, 55.0, 40.0, 39.0, 57.5, 53.5, 28.0 and 95.0 µg/ml, respectively, while standard drug ascorbic acid is 27.0 µg/ml.
Among the naturally isolated metabolites, only 5 showed better IC50 value on ferric ion (Fe3+) of 37.0 µg/ml than the ascorbic acid (41.0 µg/ ml). While except 4a, all semi-synthetic analogs showed prominent IC50 values on Fe3+ ions than the standard drug. The 50 % inhibition of Fe3+ ions of 4e, 4b, 4d and 4c were found to be 27.0, 33.5, 38.5 and 40.5 µg/ml, respectively. The IC50 values of 4, 4a, 1, 2, 3 and AE on Fe3+ ions was found to be 43.5, 46.0, 46.5, 46.5, 64.5 and 91.0 µg/ml, respectively.
Likewise, among the metabolites, only 3 and 4 showed better IC50 values on superoxide free radicals of 32.0 and 26.0 µg/ml, respectively, when compared to the ascorbic acid (35.5 µg/ ml). While semi-synthetic analogs, namely 4a, 4c, 4d, and 4e, showed prominent IC50 values on superoxide free radicals 25.5, 35.5, 35.0, and 25.0 µg/ml, respectively than the standard drug. The 50 % concentration needed for 1, 2, 5, 4b and AE to inhibit superoxide free radicals were found to be 80.0, 87.5, 69.0, 43.5 and 136.0 µg/ ml, respectively.
In vitro anti-inflammatory activity:
In general, inflammation is caused by denaturation of biological protein[25]. In the present study, AE, secondary metabolites (1-5), and analogs (4a-e) have investigated for their restraint of bovine serum albumin denaturation influenced by heat. The outcomes of the study indicated that all tested samples exhibited noteworthy anti-inflammatory action. The 50 % inhibition of 1, 2, 3, 4, 5, 4a, 4b, 4c, 4d, 4e and AE on bovine serum albumin denaturation were found to be 814, 701, 823, 664, 700, 791, 956, 900, 112, 105 and 268 µg/ml, respectively, while indomethacin with 110 µg/ml (fig. 3).
During the period of observation, no animal was lethal, so the LD50 of AE was determined to be above 2000 mg/Kg. Based on the observations of toxicological studies, the low and high dose of AE was calculated as 100 and 200 mg/Kg b.w, respectively, and standard drug, indomethacin at 100 mg/Kg b.w (single dose). The results of in vivo anti-inflammatory assay, it is observed that AE displayed a dose-dependent depletion of edema in rat paw (Table 1).
| Sample | Mean±SD of oedema at different intervals (cm) | Percentage reduction at different intervals (%) | |||
|---|---|---|---|---|---|
| 2 h | 4 h | 2 h | 4 h | ||
| AE | 100 mg/Kg b.w | 1.12±0.07* | 1.23±0.08* | 30.9 | 35.89 |
| 200 mg/Kg b.w | 1.05±0.03* | 1.08±0.04* | 40.19 | 43.82 | |
| Indomethacin | 1.18±0.08* | 1.12±0.09* | 32.89 | 41.38 | |
| Control | 1.76±0.09 | 1.91±0.09 | - | - | |
Note: ns: not significant; *p<0.05
Table 1: In Vivo Anti-Inflammatory Assay of Acetone Extract of Ramalina leiodea (AE) on Formalin-Induced Hind Rat Paw Oedema Method
The animal group treated with 100 mg/Kg b.w (lower dose) of AE displayed 30.90 and 35.89 percentage reduction of edema in rat paw at 2 and 4 h, respectively. Moreover, animals administered with 200 mg/Kg b.w (high dose) of AE presented prominent percentage reduction of edema in rat paw with 40.19 % and 43.82 %, at 2 and 4 h, respectively, when compared to indomethacin with 32.89 % and 41.38 %, respectively (Table 1). Hence, form both in vitro and in vivo anti-inflammatory assays, it can be justified that AE is effective in protein denaturation, as well as reducing edema in the rat paw.
Generally, prolonged inflammation many dangerous diseases, as well as cancer. As AE, secondary metabolites (1-5), and analogs (4a-e) revealed well anti-inflammatory actions, we additionally estimated for their anti-cancer properties by the SRB assay, using four cancer cells, namely MCF- 7, DLD-1, HeLa, FADU and A549 and one normal cell line (NHME). The results of the percentage of inhibition of cell growth against respective concentrations are designed to attain IC50 values.
At tested higher concentration (100 µg/ml), AE exhibited effective inhibitory properties against MCF-7, DLD-1, HeLa, FADU, and A549 with 94.21±1.06, 98.84±0.53, 89.96±1.41, 97.80±1.73 and 88.08±1.59, respectively, than that of the doxorubicin at 10 µg/ml. Among the metabolites, only 2 and 5 shown a sensible degree of specificity against tested cancer cells, while among the semi- synthetic analogs, only 4b and 4d demonstrated a noticeable degree of specificity against tested cancer cells. Furthermore, all tested samples revealed a low degree of specificity against NHME specifies non-lethal to normal cells.
The 50 % cell growth inhibition of AE against DLD-1, HeLa, FADU, and A549 was found to be 36.5, 30.0, 60.0, 40.0, and 42.0 µg/ml, respectively (fig. 4). Compounds 2, 5, and 4d exhibited noticeable IC50 values of 9.0, 25.5, and 26.5 µg/ ml, respectively, on MCF-7, however doxorubicin with 5.5 µg/ml. The 50 % cell growth inhibition of 2, 5, 4b, and 4d against DLD-1 was found to be 5.5,17.7, 16.0, and 17.0 µg/ml, respectively, whereas doxorubicin with 5.4 µg/ml. On HeLa, compounds 2, 5, 4b and 4d exposed IC50 value of 7.4, 18.5, 25.8, and 12.0 µg/ml, respectively, while doxorubicin with 4.5 µg/ml. The 50 % cell growth inhibition of 2, 5, 4b and 4d against FADU was found to be 5.9, 21.0, 25.0, and 14.0 µg/ml, respectively, and 7.6, 27.5, 22.3, and 21.2 µg/ml against A549, respectively, whereas doxorubicin with 3.8 and 6.3 µg/ml, respectively.
In the current research work, secondary metabolites (1-5), and analogs (4a-e), which were obtained from acetone extract of R. leiodea (AE) were exposed to in vitro studies of antioxidant (ferric ions, DPPH, and superoxide free radicals), anti-inflammatory (albumin protein denaturation method and formalin-induced rat paw edema assay) and anticancer (SRB assay) activities. Furthermore, the acute toxicity studies were performed on AE.
The in vitro assays of antioxidant and anti- inflammatory revealed that all the tested samples exhibited noticeable inhibition properties against free radicals, metal ions, and bovine serum albumin denaturation. The mechanism of action for the isolated secondary metabolites and semi-synthetic analogs for antioxidant and anti-inflammatory activities is proposed to have a high presence of liberally accessible oxygenated constituents, specifically carboxylic acids and phenolics in their natural structure (fig. 2 and fig. 3).
The median lethal dosage of AE was observed to be above 2000 mg/Kg and the dosage of AE was established as 100 and 200 mg/Kg b.w, respectively AE was determined. Based on the protein denaturation and acute toxicology studies, the AE was subjected to edema assay in rat paw using formalin. The outcomes are denoted as the percentage reduction of albino rat paw edema calculated to basal rat paw size (Table 1). From the outcomes of edema assay in rat paw assay, it is evident that AE showed a dose reliant reduction of albino rat paw edema. Besides, a higher dose of AE is extremely effective in reducing paw edema in albino rats than that of the Indo (Table 1).
From the results of the protein denaturation method, it can be found that AE, secondary metabolites (1-5), and analogs (4a-e) exhibited noticeable inhibitory abilities against bovine serum albumin protein denaturation (fig. 2). From the data, it is proposed that AE may act by blocking the biosynthesis of Interleukin-8, prostanoids, and thromboxane[26]. Hence, AE has noticeable anti- inflammatory competence against both acute and chronic simulations of inflammation connected with arthritis[28].
Moreover, as the AE, secondary metabolites (1-5), and analogs (4a-e) represented substantial anti-inflammatory possessions, we assessed them for their anti-cancer properties by SRB assay using MCF-7, DLD-1, HeLa, FADU and A549 cancer cells and NHME cells. Form fig. 4, and it can be concluded that crucial elements responsible for the anti-cancer activity of AE were 2 and 5. Moreover, the semi-synthetic analogs, namely 4b and 4d, showed a better biological profile than the parent compound. Also, as stated earlier, chronic inflammation eventually causes cancer, the anticancer action of AE and its secondary metabolites (1-5), and analogs (4a-e) was due to their antioxidant and anti-inflammatory abilities. Furthermore, AE, secondary metabolites (1-5), and analogs (4a-e) displayed a slight degree of specificity against NHME conditions indicate non-lethal to human cells.
To conclude, the study is a pharmacological evaluation report of earlier isolated five known secondary metabolites, namely usnic acid (1), ethyl everninate (2), scrobiculin (3), methyl 2,6-dihydroxy-4-methyl benzoate (4) and 2'-O-methyldivaricatic acid (5) from acetone extract of R. leiodea (AE), and their analogs (4a-e). The outcomes showed that among all the metabolites 2 and 5 exhibited inhibitory abilities against free radicals, metal ions, bovine serum albumin denaturation, edema in rat paw, and series of cancer cell lines. Moreover, the semi- synthetic analogs, namely 4b and 4d, showed a better biological profile than the parent compound. Furthermore, the key secondary metabolites accountable for R. leiodea natural action are 2 and 5.
Conflict of interests:
The authors declared no conflict of interests.
References
- Moreira AS, Braz-Filho R, Mussi-Dias V, Vieira IJ. Chemistry and biological activity of Ramalina lichenized fungi. Molecules 2015;20(5):8952-87.
[Crossref] [Google Scholar] [PubMed]
- Stark JB, Walter ED, Owens HS. Method of isolation of usnic acid from Ramalina reticulata. J Am Chem Soc 1950;72:1819-20.
- Paudel B, Bhattarai HD, Lee HK, Oh H, Shin HW, Yim JH. Antibacterial activities of Ramalin, usnic acid and its three derivatives isolated from the Antarctic lichen Ramalina terebrata. Z Naturforsch C J Biosci 2010;65(1-2):34-8.
[Crossref] [Google Scholar] [PubMed]
- Paudel B, Bhattarai HD, Koh HY, Lee SG, Han SJ, Lee HK, et al. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011;18(14):1285-90.
[Crossref] [Google Scholar] [PubMed]
- Yamamoto Y, Watanabe A. Fatty acid composition of lichens and their phyco-and mycobionts. J Gen Appl Microbiol 1974;20:83-86.
- González AG, Barrera JB, Pérez EM, Padrón CE. Chemical constituents of the lichen Ramalina hierrensis. Planta Med 1992;58:214-8.
[Crossref] [Google Scholar] [PubMed]
- Culberson CF. Some constituents of the lichen Ramalina siliquosa. Phytochemistry1965;4:951-61.
[Crossref] [Google Scholar] [PubMed]
- Tay T, Türk AÖ, Y?lmaz M, Türk H, K?vanç M. Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea and its (+)-usnic acid, norstictic acid, and protocetraric acid constituents. Z Naturforsch C J Biosci 2004;59(5-6):384-8.
[Crossref] [Google Scholar] [PubMed]
- Marshak A, Barry GT, Craig LC. Antibiotic Compound isolated from the Lichen Ramalina veticulata. Science 1947;106(2756):394-395.
[Crossref] [Google Scholar] [PubMed]
- Aslan A, Gulluce M, Agar G, Karadayi M, Bozari S, Orhan F. Mutagenic and antimutagenic properties of some lichen species grown in the Eastern Anatolia Region of Turkey. Cytol Genet 2012;46:291-6.
[Crossref] [Google Scholar] [PubMed]
- Esimone CO, Grunwald T, Nworu CS, Kuate S, Proksch P, Überla K. Broad spectrum antiviral fractions from the lichen Ramalina farinacea (L.) Ach. Chemotherapy 2009;55(2):119-26.
[Crossref] [Google Scholar] [PubMed]
- Hoskeri HJ, Krishna V, Amruthavalli C. Effects of extracts from lichen Ramalina pacifica against clinically infectious bacteria. Researcher 2010;2(3):81-5.
- Weissman L, Garty J, Hochman A. Characterization of enzymatic antioxidants in the lichen Ramalina lacera and their response to rehydration. Appl Environ Microbiol 2005;71(11):6508-14.
[Crossref] [Google Scholar] [PubMed]
- Chang YH, Ryu JS, Lee SH, Park SG, Bhattarai HD, Yim JH, et al. Inhibition of melanogenesis by Ramalin from the Antarctic lichen Ramalina terebrata. J Soc Cosmet Sci Korea 2012;38:247-254.
- Türkez H, Ayd?n E, Aslan A. Effects of lichenic extracts (Hypogymnia physodes, Ramalina polymorpha and Usnea florida) on human blood cells: Cytogenetic and biochemical study. Iran J Pharm Res 2012;11:889.
[Google Scholar] [PubMed]
- Gulluce M, Aslan A, Sokmen MÜ, Sahin FI, Adiguzel A, Agar G, et al. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine 2006;13(7):515-21.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Vedula GS. In vitro anti-inflammatory and cytotoxicity studies of two mangrove associated lichens, Dirinaria consimilis and Ramalina leiodea extracts. Hygeia JD Med 2018;10(1):16-26.
- Esimone CO, Adikwu MU. Antimicrobial activity and cytotoxicity of Ramalina farinacea. Fitoterapia 1999;70:428-431.
- Tatipamula VB, Vedula GS. Antimicrobial and anti tubercular activities of isolates and semi synthetic derivatives of lichen Ramalina leiodea (Nyl.) Nyl. J Serbian Chem Soc 2019;84(6):555-62.
- Bharadwaj V. New record of mangrove lichens from Andhra Pradesh and Orissa states of India. Studies Fungi 2019;4(1):90-3.
- Tatipamula VB, Killari KN, Ketha A, Sastry VG. Taxithelium napalense acts against free radicals and diabetes mellitus. Bangladesh J Pharmacol 2017;12:197-203.
- Haritha P, Patnaik SK, Tatipamula VB. Chemical and pharmacological evaluation of manglicolous lichen Graphis ajarekarii Patw. & CR Kulk. Vietnam J Sci Technol 2019;57:300-308.
- Talluri MR, Ketha A, Battu GR, Tadi RS, Tatipamula VB. Protective effect of Aurelia aurita against free radicals and streptozotocin-induced diabetes. Bangladesh J Pharmacol 2018;13:287-295.
- Tatipamula VB, Haritha P, Rao GSNK, Ketha A, Yejella RP. Isolation and characterization of metabolites from Clathria procera Ridley extract and evaluation of its antidiabetic effects in streptozotocin-induced diabetic rats. J Exp Appl Animal Sci 2019;3:35-56.
- Tatipamula VB, Vedula GS, Sastry AV. Antarvediside AB from manglicolous lichen Dirinaria consimilis (Stirton) DD Awasthi and their pharmacological profile. Asian J Chem 2019;31(4):805-12.
- Tatipamula VB, Vedula GS. Anti-inflammatory properties of Dirinaria consimilis extracts in albino rats. J Biomed Sci 2017;4(1):3-8.
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoco 2006;1:1112.
- Tatipamula VB, Vedula GS, Sastry AV. Chemical and pharmacological evaluation of manglicolous lichen Roccella montagnei Bel em. DD Awasthi. Future Journal of Pharmaceutical Sciences 2019;5:8.
 Superoxide
Superoxide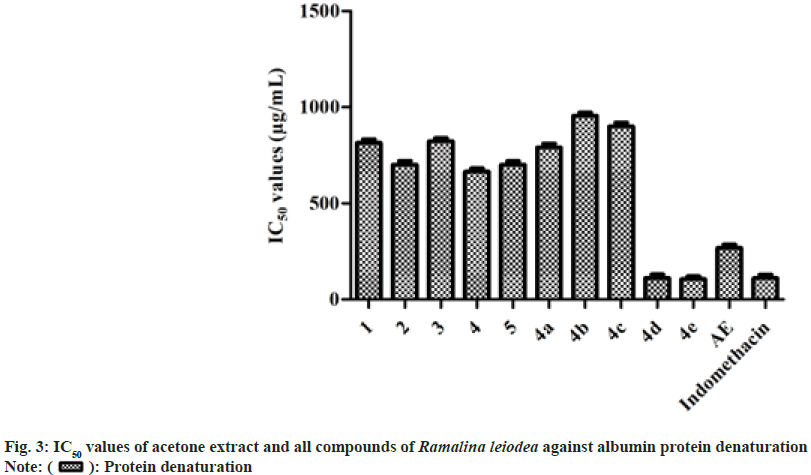
 Protein denaturation
Protein denaturation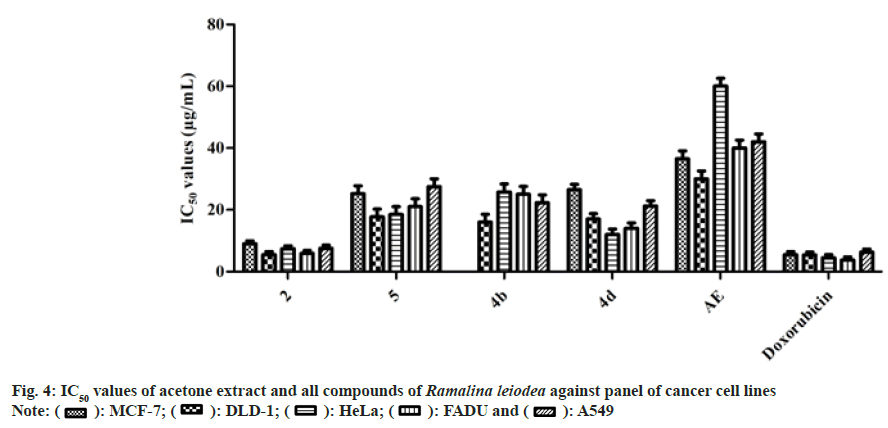
 A549
A549




