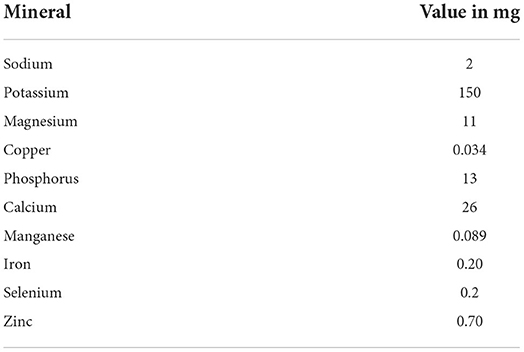Lagenaria siceraria fruit: A review of its phytochemistry, pharmacology, and promising traditional uses
- 1College of Animal Science and Technology, Northwest A&F University, Yangling, China
- 2The Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
- 3Mayo Hospital Lahore, Lahore, Pakistan
- 4Department of Physical Education, Beijing Sports University, Beijing, China
- 5District Head Quarter (DHQ) Hospital, Vehari, Pakistan
- 6Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 7Department of Veterinary Microbiology, Sindh Agriculture University, Tando Jam, Pakistan
Since ancient times, the Cucurbitaceae family is used as a therapeutic option in human medicine. This family has around 130 genera and 800 species. Researchers have studied the various plants of this family including Lagenaria siceraria due to their medicinal potential. Various properties are beneficial for human health, that have been attributed to L. siceraria like antioxidant, hypolipidemic, diuretic, laxative, hepatoprotective, analgesic, antihypertensive, cardioprotective, central nervous system stimulant, anthelmintic, free radical scavenging, immunosuppressive, and adaptogenic. The fruit of this plant is commonly used as a vegetable that has a low-calorie value. The species possess a diverse set of biological compounds like flavonoids, sterols, saponins, and terpenoids. Vitamins, choline, flavonoids, minerals, proteins, terpenoids, and other phytochemicals are also found in the edible parts of this plant. Besides 17 different amino acids, many minerals are reported to be present in the seeds of L. siceraria. According to the USDA nutritional database per 100 g of L. siceraria contains 14 Kcal energy, 3.39 g carbohydrates, 0.62 g protein, 0.2 g fat, and 0.5 g fiber. L. siceraria performs a wide range of pharmacological and physiological actions. The literature reviewed from various sources including PubMed, Science Direct, Google scholar, etc. shows the remarkable potential to treat various human and animal illnesses due to its' potent bioactive chemicals. The key objective of this thorough analysis is to present a summary of the data about the beneficial and harmful effects of L. siceraria intake on human health, as well as in veterinary fields.
Introduction
There is an emergent concern about herbal medicines across the world, which is complemented by more laboratory research into the pharmacological characteristics of bioactive substances and their capacity to cure various disorders. Through ethnopharmacology and traditional medicine, a slew of new medications has made their way onto the worldwide market (1). For centuries, herbal treatments have been used to cure and manage a variety of ailments. Herbal medications are a viable alternative to current synthetic treatments owing to their few adverse effects and are regarded as safe and useful in the treatment of human ailments. The Cucurbitaceae family includes the Lagenaria siceraria (Mol.) Standley fruit (Bottle gourd), utilized in a separate system of traditional medicine to cure numerous ailments (2). A significant number of medicinally beneficial plants belong to the Cucurbitaceae family. This family has around 130 genera and 800 species. Cucurbitacin, a secondary metabolite found in the seeds and fruit sections of several cucurbits, has been described to have purgative, emetic, and antihelmintic actions. This category of chemicals had been considered for its anti-inflammatory, hepatoprotective, cytotoxic, and cardiovascular properties (3). Bottle gourd (L. siceraria), family Cucurbitaceae is a medicinal plant whose diverse sections had been identified for their therapeutic potential. The plant's fruiting body is well-liked for its taste and extraordinary nutritional content, which includes practically all of the needed ingredients for good health. The plant might provide physiologically active polysaccharides (4). Being a domestic plant, it is for both food and medicine. Cardioprotective, antidepressant, anti-hyperglycemic, antimicrobial, cytotoxic, anti-inflammatory, antihyperlipidemic, anti-urolithiasis, antianxiety, analgesic, anticancer, diuretic, anthelmintic, antihepatotoxic, anthelmintic, antistress, immunomodulatory, antiulcer, hepatoprotective, and antioxidant activities have been studied in various parts of this plant. To emphasize the medicinal value of this plant, its phytochemical elements, and traditional, pharmacological, and medicinal applications are studied in this review. This would be beneficial in resurrecting its relevance and highlighting its many potential qualities to motivate academics to do more study on L. siceraria (2). The key objective of this thorough analysis is to present a summary of the data about the beneficial and harmful effects of L. siceraria intake on human health, as well as in veterinary fields like the poultry and livestock sector.
Botanical description
The L. siceraria (Molina) is a member of the Cucurbitaceae family and is also called Bottle gourd. It is a climbing perennial plant that is extensively grown as a vegetable crop in tropical nations such as Thailand, Egypt, India, Japan, and the rest of the world (1, 5). The fruits of Bottle gourd have a variety of shapes: they can be huge and rounded, small and bottle-shaped, or slim and serpentine, and they can grow to be over a meter long. Rounder varieties are typically called calabash gourds.
The fruit diversity and phytogeographical distribution of L. siceraria in Nigeria were studied and 24 different shapes of fruits were explored as shown in Figure 1 (6).

Figure 1. Different shapes of Lagenaria siceraria found across the world (6).
The bottle gourd is said to have originated in Africa and spread worldwide in pre-Columbian times, maybe via floating on the oceans. It moved from India to Indonesia, New Zealand, and China where it has diversified into several local kinds. It is a robust annual vine with huge leaves and a lush look that may be grown as a running or climbing vine (7) (Table 1).

Table 1. Botanical description of various parts of Lagenaria siceraria (7).
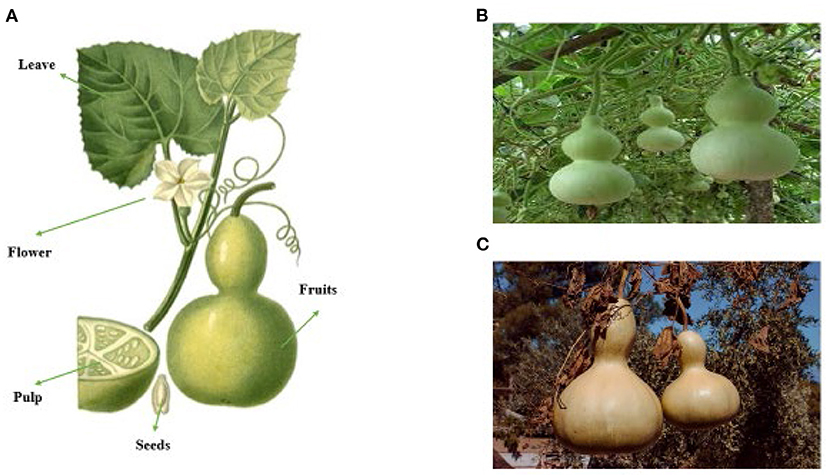
Figure 2 shows different parts of Lagenaria siceraria (A), fresh fruit (B), ripened fruit (C).
Vernacular names
Vernacular names of L. siceraria in different languages across the world are given in Table 2.
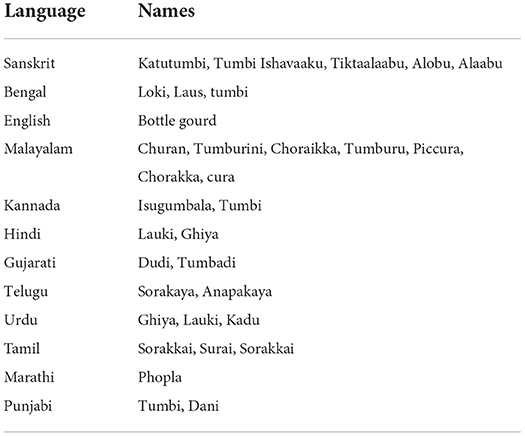
Table 2. Vernacular names of Lagenaria siceraria (8).
Traditional uses
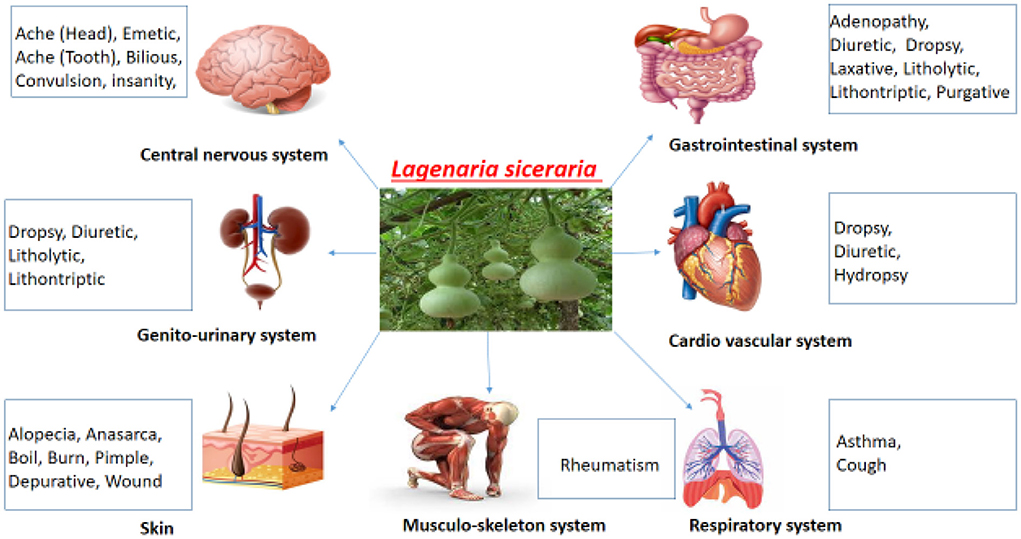
The fruit is extensively used as a medicinal vegetable in Asia and Africa for a variety of ailments. Alternative medicine is made from several components of this plant, including the fruit, seed, leaf, and root (9). In Ayurveda and other folk remedies, the plant's fruits had been noted to have possible therapeutic benefits. Traditional uses of the fruit include cardioprotective, antidote, aphrodisiac, cardiotonic, diuretic, and general tonic properties (5). The fruit juice had been a cure for jaundice and heal other liver ailments as it possessed good anti-oxidants (10). Various properties that are beneficial for human health have been attributed to this plant like antioxidant, hypolipidemic, diuretic, laxative, hepatoprotective, analgesic, antihypertensive, cardioprotective, central nervous system stimulant, anthelmintic, free radical scavenging, immunosuppressive, and adaptogenic (11). Anti-HIV, antipyretic, anthelmintic, anxiolytic, carminative, anti-diabetic, antibacterial, antioxidant, laxative, anti-tuberculosis, anti-diarrhoeal, and purgative are only a few of the therapeutic qualities of the Cucurbitaceae family. It's also used as a contraceptive, diuretic, and cardiotonic agent. Anti-inflammatory, antitussive, cytotoxic, and expectorant activities are also present (3). The diuretic efficacy of methanol and vacuum dried juice extract of the fruits had been studied. Albino rats had a larger urine volume when compared with the control group. Both of the extracts displayed a dose-dependent rise in electrolyte excretion (12). The plant species aids in improved digestion eliminates urinary difficulties, and aids in weight loss and blood pressure-lowering (13). For the treatment of illnesses and disorders in humans, L. siceraria has been utilized in several systems of traditional medicine. This vegetable has high water content and a low-calorie count. The seeds are also utilized for headaches and constipation since they have a cooling impact on the body (8).
After drying the fruit is used as resonance boxes for the kora and balafon (xylophone). Drinking water, milk, liquor, local wine, oatmeal, food grains, animal fat, honey, tobacco, ghee, salt, perfume, medicinal herbs, and crop seeds are all stored and transported in dried bottle gourd fruits. Beehives, beer-making containers, or storing clothing and cutlery are all created from dried fruit shells. Many musical instruments and beautiful decorations are made from dried bottle gourds (14). The climber's medicinal abilities have been used to cure a variety of disorders, including jaundice, ulcers, colitis, diabetes, insanity, skin problems hypertension, piles, and congestive cardiac failure (CCF). The fruit pulp has cooling, diuretic, antibilious, and pectoral qualities, and is used as an emetic and purgative. This pulp boiled in oil is used in treating rheumatism and sleeplessness (15).
Traditional uses of various parts of L. siceraria are given in Table 3 (16).

Table 3. Traditional uses of different parts of Lagenaria siceraria (16).
Phytochemistry
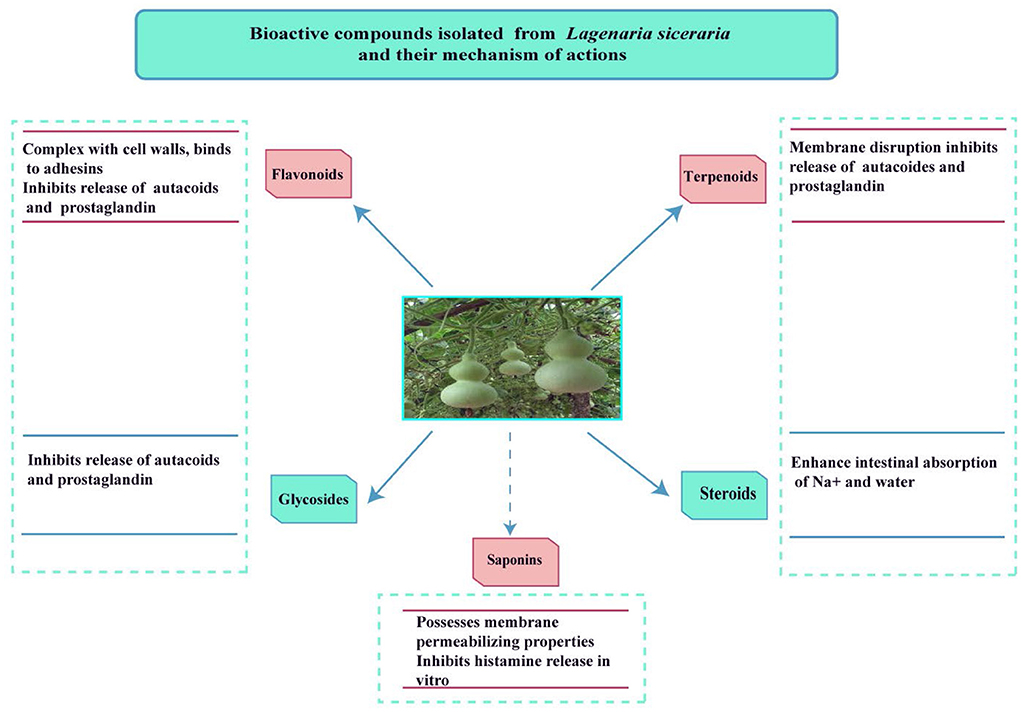
Ascorbic acid, triterpenes, minerals, choline, amino acids, vitamin-B complex, triterpenoid cucurbitacins B, D, H, G, 22-deoxy cucurbitacin, β-glycosidedase-elasterase, flavonoids, sterols, and carbohydrates are all found in the edible part of the fruit (5).
Cucurbitacins B, H, G, and D, as well as the bitter principle of the Cucurbitaceae, are said to be present in the fruit along with Flavone-C glycosides (a ribosome-inactivating protein), two sterols, i.e., fucosterol and campesterol, terpene byonolic acid (an allergic compound) and Lagenin (1).
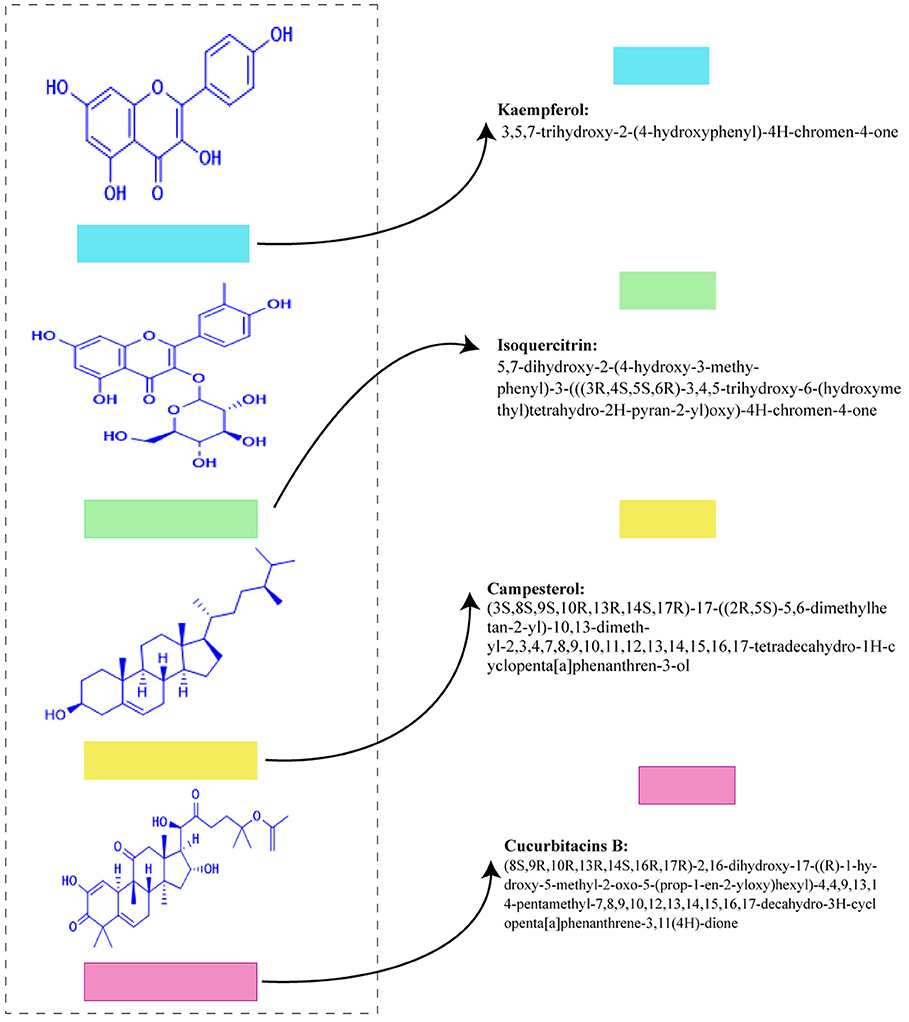
Figures 3, 4 shows the phytochemicals of Lagenaria siceraria.
The extract has carbohydrates, saponins, proteins, flavonoids, and glycosides as shown by the phytochemical test (17). This vegetable has high water content and a low-calorie value. Vitamins, choline, flavonoids, minerals, proteins, terpenoids, and other phytochemicals are found in the edible section. L. siceraria contains a variety of bioactive chemicals, including flavones, sterols, cucurbitacins, C-glycosides, triterpenoids, and -glycosides (2).
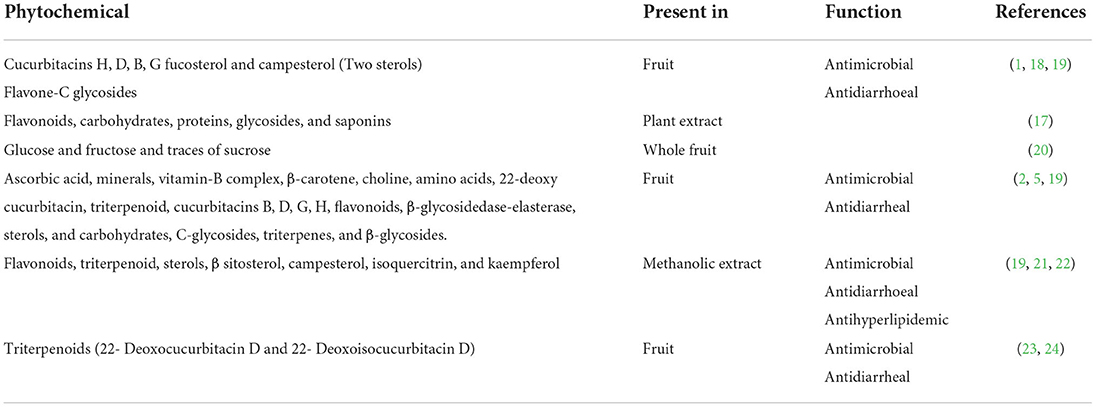
Table 4 shows phytochemicals and their functions present in different parts of Lagenaria siceraria.
Nutritional profile
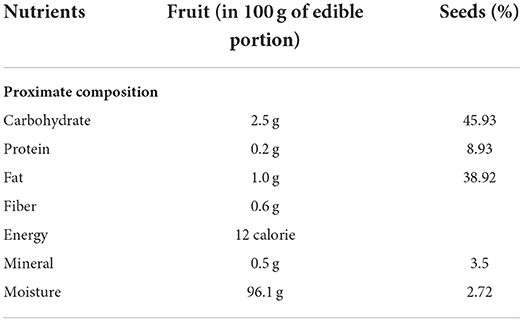
Seeds contained 45.0–47.8 g/100 g crude fat, 8.1–7.3 g/100 g carbohydrates, 37.2–35.0 g/100 g crude protein, and 4.0 g/100 g moisture (25).
Nutrients
The nutrient composition of L. siceraria (fruit and seeds) is given in Table 5.
The USDA (United States Department of Agriculture) nutritional database exhibited that each 100 g of L. siceraria has 14 Kcal energy, 3.39 g carbohydrates, 0.62 g protein, 0.2 g fat, and 0.5 g fiber (27).
Minerals
Calcium, Potassium, Magnesium, Lead, Iron, Sodium, Zinc, and chromium were found in the seeds of L. siceraria fruit (26). Furthermore, Phosphorus, Copper, Manganese, and Cobalt were also reported in this plant (28).
Table 6 shows numerous minerals present in L. siceraria.
Amino acids
Seventeen amino acids lysine, methionine, threonine, proline, cysteine, glutamic acid, phenylalanine, arginine, tyrosine, histidine, valine, serine with glutamic acid, alanine, leucine, isoleucine, aspartic acid, glycine, leucine, and aspartic acid were found in seeds of L. siceraria (28).
Health effects of L. siceraria
Anti-inflammatory properties
In rats and mice, L. siceraria's ethanolic extract (fruit and leaves) was tested for anti-inflammatory and analgesic properties. Carrageenan-induced edema, tail immersion pain, and acetic acid-induced writhing models were used to investigate the extract's activity. On the writhing test, the extract exhibited strong anti-inflammatory and analgesic potential. The extract comprises flavonoids, carbohydrates, proteins, glycosides, and saponins, according to a phytochemical analysis (17).
Anti-oxidant properties
The aqueous extract of L. siceraria has a strong scavenging action, and the high phenolic content of calabash fruit may help to alleviate the oxidative stress associated with diabetes (29). Antioxidant activity is recognized for phenolics and flavonoids, which have a remarkable capacity to scavenge free radicals created in human bodies. As a result, determining the number of phenolics and flavonoids in a plant sample can help determine its antioxidant capacity. The antioxidant capacity of the pedicles of L. siceraria fruits was investigated in vitro. The ethyl acetate fraction even at low concentrations showed the most effective DPPH radical scavenger (30). In vitro, a methanol extract of the aerial section of L. siceraria was reported to scavenge DPPH, hydrogen peroxide, superoxide radical, and nitric oxide as well as prevent lipid peroxidation in a concentration-dependent way. The antioxidant action of MELS (methanol extract of L. siceraria aerial parts) was attributed to its high phenolic and flavonoid content (31). In vitro experiments such as the reducing power assay, radical scavenging assay, superoxide scavenging assay, lipid peroxidation inhibition assay, and the ethyl acetate extract of bottle gourd were shown to exhibit high antioxidant activity. The quantity of phenolic compounds found in bottle gourd extracts is proportional to their radical scavenging action (32). In an isolated rat heart model, the extract's antioxidant capacity was measured in terms of glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), vitamin C (Vit C), glutathione reductase (GR), reduced glutathione (GSH), vitamin E (Vit E), and glutathione S-transferase (GST). The activities of enzyme antioxidants such as CAT, GSH, GR, and SOD were significantly reduced in isoproterenol-induced rats. It may be determined that L. siceraria's ethanolic extract has antioxidant effects (33). Extraction of seeds with ethanol resulted in a significant number of phytochemicals and antioxidant activity. All of these phytochemicals are powerful reducing agents, metal chelators, and radical scavengers, and they may be to blame for the seeds' high antioxidant activity. The methanolic extract of seeds showed good DPPH and radical scavenging capabilities in antioxidant tests (34). In human patients with dyslipidemia, the effects of L. siceraria fruit extract were investigated. The antioxidant potential of L. siceraria fruit extract was demonstrated in dyslipidemic patients by increases in Superoxide dismutase and Glutathione levels (35).
Anti-cancer properties
The study aimed to see the effect of methanol extract of L. siceraria aerial parts on anti-cancer properties. In mice, Ehrlich's Ascites Carcinoma model. The effect of medication response was assessed using the research of tumor growth response, which included an increase in life duration, a study of hematological parameters, biochemical estimates, and a liver tissue antioxidant assay. The cytotoxicity and antioxidant capabilities of L. siceraria, as well as the flavonoid content of the methanol extract of L. siceraria aerial parts, demonstrated that L. siceraria has strong anticancer activity (36). The antitumor effectiveness of L. siceraria fruit was investigated using human cancer cell lines (MCF-7 and HT-29). With varied potency and selectivity, the bitter component of L. siceraria displayed substantial anticancer action against both cancer cell lines. Cucurbitacin I and other bioactive compounds in L. siceraria fruit bitter extracts had dose-dependent inhibitory and cytotoxic effects on tested cell lines, which can be ascribed to the presence of cucurbitacin I and other bioactive compounds in L. siceraria fruit bitter extracts (34). A methanolic extract of L. siceraria Standley Fruit was tested for anti-mutagenic properties. The anti-mutagenicity of plant extracts ranged from low to high. The Ames test was employed in this investigation to assess the antimutagenic activity of direct (Sodium azide) acting mutagens in Salmonella typhimurium strains TA98 and TA100. The study found that the TA98 and TA100 strains have considerable antimutagenicity against mutagen. The antimutagenicity of the extract discovered in this investigation suggests that L. siceraria Standley Fruit has chemopreventive pharmacological relevance, which is attributable to its anti-oxidant potential (37). In Swiss albino mice, the chemopreventive efficacy of bottle gourd juice (BGJ) against cutaneous papilloma genesis was investigated. The chemopreventive properties of bottle gourd against skin cancer were demonstrated by a reduction in papilloma number, multiplicity, incidence, latency, volume, and papilloma size, as well as histological examinations. The presence of phytochemicals working through several pathways might be responsible for the protective benefits (38, 39). On lung cancer cell lines, the cytotoxic effects of plant fruit extract were investigated. According to the findings, the presence of cucurbitacin, polysaccharide inhibitor lagenin, and flavonoids in this plant's extract had a significant growth inhibitory influence on the lung cancer cell line (40). Immune-potentiating action is induced by the latex sap of dietary L. siceraria (LSL), which has high lectin activity. In both in vitro and in vivo tumor models, LSL inhibits tumor cell growth. Through changed gene expression, it suppresses tumor growth by targeting apoptosis and tumoral neovasculature. Its possible mechanism of action is given in Figure 5 (41).
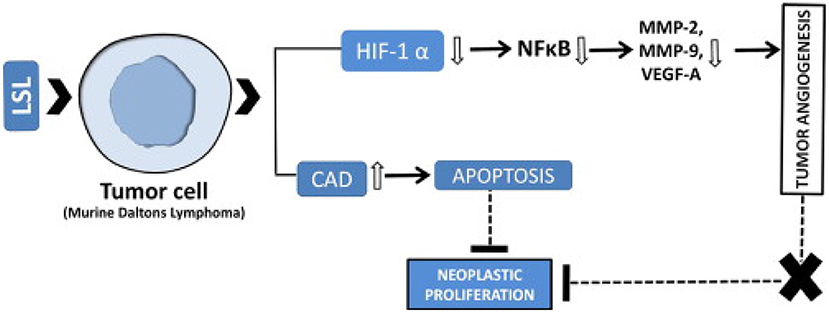
Figure 5. Anti-cancerous mechanism of action of Lagenaria siceraria (41).
Anti-obesity properties
Some fatty acid esters were found in the chloroform fraction of L. siceraria, including isopropyl palmitate, 9,12- octadecadienoic acid methyl ester, hexadecanoic acid methyl ester, and alpha-linolenic acid methyl ester. L. siceraria's ability to decrease pancreatic lipase activity, reducing lipid breakdown and hence lowering fat entrance into the body, is due to these chemicals. Regular consumption of the fruit's aqueous decoction may therefore be suggested for weight loss. Fatty acids and their esters acted as lipase inhibitors (42). In high-fat diet-generated obese mice, the synergistic impact of Commiphora Mukul (Gum Resin) with L. siceraria (fruit) extracts was examined. After the combined treatment, there was a substantial reduction in body weight, triglyceride, VLDL levels, fasting blood glucose, LDL, and serum cholesterol, as well as an increase in HDL levels. The results showed that combining C. Mukul and L. siceraria reduced obesity caused by a high-fat diet (43). The effects of L. siceraria fruit extract on human disease patients were investigated. There were significant decreases in body mass index (35). Obesity in Wistar albino rats was created by feeding them a high-fat diet and were treated with a diet containing L. siceraria, there was a significant reduction in body weight, locomotor activity, total cholesterol, food intake, triglycerides, organ weights, and an increase in low and high-density lipoprotein levels, indicating that L. siceraria has anti-obesity potential. The aqueous extract included numerous chemical ingredients such as saponins, pectin, and ellagic acid, which are essential for decreasing body weight and cholesterol levels, according to the preliminary phytochemical assessment of LS and TA. The LS fruits are high in saponins, cucurbitacins B, G, D, H, triterpenoid, and pectin, which showed lipid-lowering properties (44, 45). Different bottle gourd extracts were tested for their antihyperlipidemic and hypolipidemic properties in Triton-induced hyperlipidemic rats, as well as their hypolipidemic effects in normocholesterolemic rats. The extracts lowered total cholesterol, triglycerides, and low-density lipoproteins levels in a dose-dependent manner, while dramatically increasing high-density lipoproteins levels. The effects of petroleum ether extract were not significant. When compared to the others, the chloroform and alcoholic extracts had more substantial impacts on triglycerides, total cholesterol, and low-density lipoproteins, as well as an increase in HDL (46). The antihyperlipidemic activity of a methanolic extract of L. siceraria fruits (LSFE) was tested in high-fat-diet-induced hyperlipidemic rats. When compared to rats fed a high-fat diet, the gain in weight in rats given LSFE was smaller. Furthermore, LSFE caused a considerable increase in bile acid excretion. It may work by affecting endogenous cholesterol production in the liver and boosting cholesterol end product excretion. The LSFE included flavonoids, saponins and steroids, and polyphenolics, according to preliminary phytochemical screening. Plant saponins and steroids have been shown in several investigations to have hypolipidemic and antihyperlipidemic properties (47). The juice of L. siceraria (Bottle gourd) includes all of the active components that inhibit fat storage in adipose tissue. The anti-obesity activity of L. siceraria (Bottle gourd) juice has been tested in overweight and obese human individuals. Weight, waist circumference, and BMI all decreased significantly in those who consumed Bottle gourd juice, indicating it is a safe and effective treatment option for obese people (48).
Immunity boosting properties
The immunomodulatory impact of a methanolic extract of L. siceraria fruits in rats was investigated. The different fractions of L. siceraria were given orally at dosages of 100, 200, and 500 mg/kg to rats resulting in a great reduction in the delayed-type hypersensitivity reaction. Both primary and secondary antibody titers increased in a dose-dependent manner. Fractions also enhanced the number of white blood cells and lymphocytes. The findings show that test fractions have immunomodulatory potential (49).
Anti-diabetes properties
In vitro, the aqueous fraction of L. siceraria fruit pedicles has significant alpha-amylase inhibitory activity. This exercise is employed as a blood glucose management method. The conversion of starch to glucose is slowed when pancreatic alpha-amylase is blocked in the small intestine. As a result, less glucose is generated and enters the bloodstream, allowing it to be employed as an anti-diabetic drug (30). Supplementing MELS enhanced lipid metabolism and functions as a preventive mechanism against the formation of atherosclerosis in diabetic rats, as well as reducing diabetic sequelae from lipid peroxidation by boosting antioxidant status. As a consequence, the aerial sections of L. siceraria methanol extract can be considered a rich source of anti-diabetic medicines, possibly due to the extract's flavonoid and polyphenolic content (50). The pulp and seed extracts of L. siceraria induced alterations in the functional status of pancreatic cells. In rats with alloxan-induced diabetes, the capacity of the organism to generate and release insulin rose at the same time as the glucose level in the blood declined. The pulp and seed extract of L. siceraria were shown to have considerable anti-diabetic action (51). The oral glucose tolerance test was used to assess the hypoglycemic qualities of different globulins extracted from male Wistar rats, and it revealed that L. siceraria seeds contained globulins with high anti-hyperglycemic activity. In the profile, there was a prominent protein band with a molecular weight of 24.61 kDa that had considerable anti-hyperglycemic action. This specific protein, if present, is most likely the active peptide responsible for the observed activity (52). In human patients with dyslipidemia, the effects of L. siceraria fruit extract were investigated. Fasting blood glucose levels were found to be significantly lower (35). Various in-vitro approaches, such as amylolysis kinetics, and glucose adsorption diffusion capacity, were used to assess the hypoglycemic efficacy of the phyto-material extracts. The suppression of an enzyme (alpha-amylase) by L. siceraria, which restricts starch to glucose conversion, was blamed for the slowing of glucose diffusion. The extracts of L. siceraria have been shown to have hypoglycemic action in several in-vitro tests and might be employed as therapeutic agents in the treatment of diabetes (53). The presence of bioactive molecules and amylase and glucosidase inhibitors, and cholinergic esterase enzymes in ethanol and methanol seed extracts of L. siceraria might explain the excellent antidiabetic action found (34).
Cardio-protective properties
Ethanolic extract has a cardioprotective effect. The antioxidant function of L. siceraria (Mol) fruits is most likely due to its capacity to combat free radicals, or its ability to maintain the near-normal activity of free radical enzymes, which protect the cardiac membrane from oxidative damage by lowering lipid peroxidation (33).
Modern pharmacological research has revealed that the fruit of L. siceraria has a variety of cardioprotective characteristics. In rats with triton-induced hyperlipidemia, chloroform, and alcoholic extracts of L. siceraria revealed antihyperlipidemic potential. In Doxorubicin and Isoproterenol-induced cardiotoxicity in rats, the fruit demonstrated strong cardioprotective benefits (5). The goal of the study was to see if L. siceraria (LS) fruit powder might protect rats against the cardiotoxicity of the drug doxorubicin (Dox). The LS-treated group was shown to be protected from doxorubicin-induced cardiac damage in histopathological analysis. It was discovered that L. siceraria had a cardioprotective effect in rats when they were exposed to doxorubicin-induced cardiotoxicity (54). The lack of cardiac necrosis and inflammation in the L. siceraria-treated group showed that the plant had cardioprotective properties. The antioxidants orientin and isoorentin found in L. siceraria fruit powder appear to help prevent cardiac necrosis and inflammation. As a result, it may be inferred that LS fruit has cardioprotective properties (55). By preserving endogenous antioxidants and reducing lipid peroxidation in the rat heart, L. siceraria (Cucurbitaceous) Fruit Juice reduced Doxorubicin-induced cardiotoxicity and lowered myocardial damage (56). In isoproterenol-induced myocardial infarction, the cardioprotective benefits of L. siceraria fruit juice were investigated. The results show that L. siceraria fruit juice has a cardioprotective effect in rats with isoproterenol-induced myocardial infarction. The presence of polyphenolic components in LS fruit may be responsible for these effects (57). Isoprenaline-induced tachycardia was decreased when L. siceraria fruit powder was given. Isoprenaline cardiotoxic impact was reduced in LS-pretreated mice. The cardioprotective effect of LS in isoprenaline-induced cardiotoxicity appears to be aided by its antioxidant and anti-inflammatory properties (58).
Gastro-protective properties
The anti-ulcer efficacy of a methanolic extract of L. siceraria fruits was examined in Wistar rats using pylorus ligation, Asprin, cold-restraint stress, and ethanol ulcer models. MELS reduced stomach volume, free acidity, ulcer index, and total acidity significantly, indicating that L. siceraria fruit extract may have anti-ulcer action (59). L. siceraria showed an increase in Gastric juice pH, whereas decreased in Total acidity, Gastric content, and Gastric juice volume. As a result of histological assessment investigations, it was shown that L. siceraria is both safe and effective in the treatment of stomach ulcers (60).
Hepato-protective properties
Based on improvements in serum marker enzyme levels, antioxidant parameters, and histological investigations, ethanolic extract of L. siceraria fruit is said to have a high hepatoprotective and antioxidant effect in antitubercular drugs induced hepatotoxicity (61).
L. siceraria has been shown to prevent the elevation of hepatic enzymes caused by long-term carbamazepine administration in rabbits, as well as liver tissue histology showing no necrosis or cholestasis. Thus, it is concluded that L. siceraria has a hepatoprotective effect and reduces the hepatotoxicity caused by carbamazepine (62). On rats, the hepatoprotective efficacy of L. siceraria fruit extracts was evaluated against carbon tetrachloride (CCl4)-induced hepatotoxicity. The toxic effect of CCl4 was dramatically reduced in L. siceraria ethanol extract-treated rats by restoring serum bilirubin, protein, and enzyme levels. The existence of normal hepatic cords, lack of necrosis, and fatty infiltration in the liver sections of the animals treated with the extracts further demonstrated the hepatoprotective potential (63). In paracetamol-induced hepatotoxicity in rats, the presence of phenolic components in ethanol extract of L. siceraria fruit protected against oxidative damage and liver necrosis (10).
Other pharmacological effects
L. siceraria (Molina) Standl. is a traditional medicinal as well as a portion of vegetable food. Immunomodulatory, antioxidant, hepatoprotective, anti-stress, cardioprotective, adaptogenic, anti-inflammatory, antihyperlipidemic, and analgesic activities have all been described. Lagenin (20 kDa), a new protein isolated from seeds, has been shown to have anticancer, antiviral, antiproliferative, and anti-HIV properties (64). Antioxidant, antihypertensive, hepatoprotective, cardioprotective, laxative, diuretic, central nervous system stimulant, adaptogenic, immunosuppressive, hypolipidemic, analgesic, anthelmintic, and free radical scavenging properties have also been proposed for the plant. This pulp boiled in oil is used in treating rheumatism and sleeplessness (16). Polysaccharides extracted from various portions of the plant have been found to have immune-modulating, anti-inflammatory, anti-tumor, cardioprotective, antioxidant, hepatoprotective, anti-diabetic, anti-hyperlipidemic, and analgesic activities in the last three decades. Several polysaccharides isolated from diverse sections of L. siceraria have been proposed with a variety of structures. Apart from its various bioactive qualities, this plant has the capacity to detoxify soil from heavy metals through the process of bioremediation (4). Antianxiety, antihyperlipidemic, diuretic, cytotoxic, cardioprotective, anti-inflammatory, antiulcer, analgesic, anticancer, antimicrobial, antidepressant, anti-hyperglycemic, anthelmintic, anti-urolithiatic, hepatoprotective, anthelmintic, immunomodulatory, antistress, and antioxidant activities have been studied in various parts of this plant (fruit, leaves, flowers, and roots) (2). Antimicrobial activity of L. siceraria extracts against Enterococcus faecalis, Salmonella typhi, Staphylococcus aureus, Klebsiella pneumonia, E. coli, and antifungal strains (Aspergillus flavus, Trichoderma harzianum, and Aspergillus oryzae) was moderate to strong (65). Fruits of L. siceraria have the ability to promote bile salt excretion, and their supplementation lowered fat levels in rats over time (47). Avinash et al. (66) described the antiulcer effect of L. siceraria. Long-term administration of L. siceraria fruit powder was done in dexamethasone-induced rats. According to the findings, hypertension activity in rats has decreased (58). L. siceraria crude methanol extract has antihelmintic efficacy against the Indian earthworm Pheretima posthuma. As a result, the leaves of L. siceraria are thought to have significant anthelmintic action (32). Adedapo et al. (67) explored the effects of the leaf extract on carrageenan- and histamine-induced paw edema in rats with swollen paws. In addition, the authors used mice to conduct acetic acid writhing and formalin tests. In rats, the scientists found a substantial reduction in paw edema, licking duration, and frequency. It has antihyperlipidemic, cardioprotective, hepatoprotective, diuretic, antidiabetic, and antihyperlipidemic effects (8). Antioxidant, antimicrobial, central nervous system activity, bronchospasm protective, antihyperglycemic, antidiarrheal activity, hepatoprotective activity, analgesic, antihelmintic activity, anti-inflammatory activity, cardioprotective effects, cytotoxic, antidiabetic, anticancer, antihyperlipidemic activity, immunomodulatory effect, and diuretic activity were discovered in the plant (68). The analgesic efficacy of methanolic and aqueous extracts of the fruit was investigated using the tail immersion method in rats to provide scientific validity to the folkloric medicinal usage of L. siceraria. The methanolic extract has a moderate activity, whereas the aqueous extract has a high activity, according to the pain threshold test. This backs up the plant's historic usage in painful and inflammatory illnesses (69). Zinc oxide nanoparticles made from L. siceraria extract were reported for antimicrobial, anti-dandruff, and anti-arthritic properties (70).
Various extracts from the leaves and stems of L. siceraria were tested for their ability to repel the Culex pipiens L. mosquito, and it was concluded that these extracts could be developed as commercial products as an effective protection measure against mosquito bites, and thus control infections transmitted by a mosquito (71). The activity of L. siceraria leaves aqueous extract and silver nanoparticles (AgNPs) produced by L. siceraria against immature stages of C. pipiens and A. phronesis was examined. For 24 h, immature stages of both mosquito species were exposed to 400, 300, 200, 100, and 50 ppm aqueous extract of L. siceraria leaves and 5, 10, 20, 30, and 40 ppm L. siceraria generated AgNPs. The results exhibited that, AgNPs generated by L. siceraria were more harmful to mosquito species examined than the aqueous extract of L. siceraria leaves (72). The effect of manufactured zinc oxide nanoparticles (ZnO NPs) made from zinc nitrate and aqueous peel extract of L. siceraria on malaria prevention was investigated. The extract of L. siceraria and its mediated ZnO NPs were tested on An. stephensi III instar larvae. The influence of the ZnO NPs-based therapy on the histology and morphology of mosquito larvae was also studied. Poecilia reticulata (P. reticulata) had a 44% predation efficiency against An. Stephensi larvae in a normal laboratory environment, but 45.8 and 61.13% predation efficiency against An. Stephensi larvae in aqueous L. siceraria extract and its mediated ZnO NPs contaminated environment, respectively. ZnO NPs produced by L. siceraria were tested against Plasmodium falciparum CQ-sensitive strains. With an IC50 value of 62.5 g/mL, the L. siceraria extract and its induced ZnO NPs displayed cytotoxic effects against HeLa cell lines. According to the findings, L. siceraria peel extract and L. siceraria-produced ZnO NPs are viable green choices for fighting malarial vectors and parasites (73). Figure 6 shows the mechanism of ZnO NPs on Plasmodium falciparum.
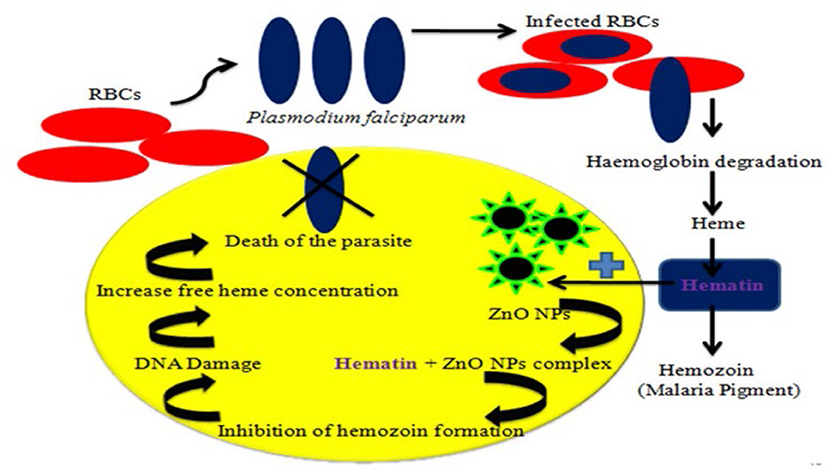
Figure 6. Schematic representation of the mechanism of ZnO NPs (nano-particles from LS) on Plasmodium falciparum (73).
Using a forced swim (behavior despair) paradigm, the antidepressant effect of methanolic extract of L. siceraria fruits in rats was assessed. The extract was given orally at dosages of 50, 100, and 200 mg/kg. The extract has antidepressant properties that are dosage dependent. The occurrence of triterpenoids, flavonoids, sterols, and saponins may be responsible for the action (23).
L. siceraria (LS) fruit juice has been used to treat jaundice and certain liver problems (10). The fruit is used to treat pain, ulcers, and fevers, as well as chest cough, asthma, and other bronchial problems, particularly in the form of a syrup made from sensitive fruits (1). Antioxidant, cardioprotective, hypolipidemic, diuretic, antihypertensive, hepatoprotective, analgesic, anthelmintic, free radical scavenging, immunosuppressive, central nervous system stimulant, laxative, and adaptogenic properties have also been proposed for the plant (11). Anti-inflammatory, antitussive, cytotoxic, and expectorant activities are also present in the plant (3). The plant species aids in improved digestion eliminates urinary difficulties, and aids in weight loss and blood pressure lowering (13). The seeds are used to treat headaches and constipation because they have a cooling impact on the body (8). The plant has long been renowned for its medicinal virtues, and it has been used to cure a variety of disorders, including diabetes, hypertension, ulcers, insanity, piles, jaundice, colitis, and skin infections. Its fruit pulp has cooling, diuretic, antibilious, and pectoral qualities, and is used as an emetic and purgative. This pulp is boiled in oil and used to treat rheumatism and sleeplessness (15).
The effect of L. siceraria on multiple systems of the human body is shown in Figure 7.
Uses in poultry and veterinary
Medicinal plants are very popular to improve the health and productivity of farm animals (67–69). The in vitro anthelmintic activity of crude aqueous methanolic extract (CAME) of L. siceraria against Haemonchus (H.) contortus and their eggs was evaluated using an adult motility assay and an egg hatch test. The in vivo anthelmintic activity of various dosages of crude powder (CP) and CAME in sheep naturally infected with gastrointestinal helminths was investigated using the fecal egg count reduction assay. CAME has significant antihelmintic action in vitro and inhibited the hatching of H. contortus eggs (71). In sheep infected with Moniezia and Avitellina species, the anticestodal action of L. siceraria seeds was examined. The powdered seeds, as well as their extracts in water and methanol, were administered orally at various doses. On the 15th day following administration, the medication powder induced a decrease in egg per gram counts (23). The purpose of the study was to develop low-salt, high-fiber, and low-fat functional chicken nuggets by substituting bottle gourd for sodium chloride and observing the effects on physicochemical parameters textural, color values, lipid, and sensory properties of pre-standardized low-fat chicken nuggets. The results of this investigation revealed that substituting bottle gourd for sodium chloride has a substantial impact on a variety of product quality parameters. Salt substitution, on the other hand, did not affect sensory qualities. Excluding the taste and quality ratings, which were impacted at greater levels, the sensory qualities of low-salt, low-fat chicken nuggets with bottle gourd were equivalent to the Control. With the addition of bottle gourd, the dietary fiber content of the goods may be increased, while the total cholesterol level can be reduced. As a result, using this technique, extremely palatable low-fat, low-salt, and high-fiber functional chicken nuggets may be developed without sacrificing their acceptability (74).
Toxicity assessment
L. siceraria is found to cause problems in the upper gastrointestinal system. The consumption of L. siceraria causes nausea, vomiting, gastrointestinal bleeding, abdominal pain, and hematemesis (20). It is also said to cause gastrointestinal toxicity along with gastric erosion (first stage of ulcer) and ulcers (21, 22). This toxicity may be attributed to the presence of triterpenoid cucurbitacins (24). This data is based on clinical data but no dose of toxicity has been mentioned. Recently, it has come to light that drinking bottle gourd juice with a bitter flavor, can have extremely hazardous responses and result in symptoms like abdominal discomfort, vomiting, diarrhea, shock, and death (75).
A little number of cucurbitacins, specifically the types including B, D, G, and H, are present in bottle gourd fruit. Cucurbitacin concentrations often don't go above 130 ppm (76). The binding of cortisol to glucosteroid receptor is inhibited by Cucurbitacins in He La cells at 37°C which depicts a strong correlation with cytotoxic activity (77). The capillary permeability is enhanced by Cucurbitacin D (78) which is associated with a persistent fall in blood pressure and accumulation of fluid in thoracic and abdominal cavities in mice.
Conclusion and future perspective
The present review gives a thorough insight into L. siceraria phytochemistry along with pharmacology, beneficial effects, medicinal uses, and limitations that suggest its' therapeutic potential. The L. siceraria has various critical health-promoting benefits such as neurological, physiological, and blood biochemical changes. Though the mechanism of action for phytochemicals may differ among various species and is not fully understood, therefore, need to be exploited. Further research is also warranted to uncover and record relevant markers (bio and molecular) that are responsible for a wide range of L. siceraria health benefits in humans, animals, and poultry.
Author contributions
MS and MK drafted the article. JB, KA, AM, and MA downloaded the articles. UY, AK, and ZM edited the article, while SC gave the main idea. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thankful to 2021YFF1000602/National Key Research and Development Program of China 2021NY-020/Key Research and Development Projects in Shaanxi Province, U1804106/Joint Funds of the National Natural Science Foundation of China, 2015CB943102/Major National Scientific Research Projects, funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hussein MMA, Arisha AH, Tayel EM, Abdo SA. Effect of long-term oral exposure to carmoisine or sunset yellow on different hematological parameters and hepatic apoptotic pathways in mice. J Anim Health Prod. (2021) 9:80–6. doi: 10.17582/journal.jahp/2021/9.s1.80.86
2. Zahoor M, Ikram M, Nazir N, Naz S, Batiha GE-S, Kamran AW, et al. A comprehensive review on the medicinal importance; biological and therapeutic efficacy of Lagenaria siceraria (Mol.) (bottle gourd) Standley fruit. Curr Top Med Chem. (2021) 21:1788–803. doi: 10.2174/1568026621666210701124628
3. Saboo SS, Thorat PK, Tapadiya GG, Khadabadi SS. Ancient and recent medicinal uses of cucurbitaceae family. Int J Ther Appl. (2013) 9:11–9. Available online at: https://npaa.in/journal-ijta/admin/ufile/1373007067IJTA_9_11-19.pdf
4. Chakraborty I, Ghosh K. Nutritional potential, health effects and structural diversity of bioactive polysaccharides from Lagenaria siceraria: a review. J Adv Sci Res. (2020) 11:34–42. Available online at: https://www.sciensage.info/index.php/JASR/article/view/504
5. Upaganlawar A. Lagenaria siceraria (Bottle Gourd) in various cardiovascular complications. Cardiovasc Dis. (2017) 1:44–56. doi: 10.2174/9781681084893117010004
6. Awala FO, Ndukwu BC, Agbagwa IO. Phytogeographical distribution and fruit diversity of Lagenaria siceraria species in Nigeria. Am J Plant Sci. (2019) 10:958–75. doi: 10.4236/ajps.2019.106069
7. Stephens JM. Gourd, Bottle– Lagenaria siceraria (Mol.) Standl. University of Florida Cooperative Extension Service, Institute of Food Agriculture Sciences (EDIS) (1994). Available online at: http://edis.ifas.ufl.edu/pdffiles/mv/mv06900.pdf
8. Kumar D, Sharma C, Singh B, Singh D. Pharmacognostical, phytochemical and pharmacological profile of natural remedy Lagenaria siceraria (Mol.) Standly: a review. Brit J Pharm Res. (2015) 7:340–52. doi: 10.9734/BJPR/2015/17641
9. Kumari N, Tajmul M, Yadav S. Proteomic analysis of mature Lagenaria siceraria seed. Appl Biochem Biotechnol. (2015) 175:3643–56. doi: 10.1007/s12010-015-1532-3
10. Panchal CV, Sawale JA, Poul BN, Khandelwal KR. Hepatoprotective activity of Lagenaria siceraria (Molina) Standley fruits against paracetamol induced hapatotoxicity in mice. Int J Pharm Sci Res. (2013) 4:371–7. doi: 10.13040/IJPSR.0975-8232.4(1).371-77
11. Yash P, Gill NS, Amber P. An updated review on medicinal properties of Lagenaria siceraria. Inter J Univ Pharm Biol Sci. (2014) 3:362–76. Available online at: http://www.ijupbs.com/Uploads/30.%20RPA1415114115.pdf
12. Ghule BV, Ghante MH, Yeole PG, Saoji AN. Diuretic activity of Lagenaria siceraria fruit extracts in rats. Indian J Pharm Sci. (2007) 69:817–9. doi: 10.4103/0250-474X.39441
13. Roopan SM, Devi Rajeswari V, Kalpana VN, Elango G. Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview. Appl Microbiol Biotechnol. (2016) 100:1153–62. doi: 10.1007/s00253-015-7190-0
14. Lim TK. Lagenaria siceraria. In: Edible Medicinal and Non-medicinal Plants, Vol. 1. Dordrecht; New York, NY: Springer (2012). p. 298–313.
15. Prajapati RP, Kalariya M, Parmar SK, Sheth NR. Phytochemical and pharmacological review of Lagenaria siceraria. J Ayurveda Integr Med. (2010) 1:266–72. doi: 10.4103/0975-9476.74431
16. Tyagi NTN, Sharma GNSGN, Shrivastava BSB. Medicinal value of Lagenaria siceraria: an overview. Int J Indig Herbs Drugs. (2017) 2:36–43. Available online at: https://www.saap.org.in/journals/index.php/herbsanddrugs/article/view/50
17. Deshmukh DB, Sherkar MR. Evaluation of in vivo analgesic and anti-inflammatory activity of ethanolic extract of medicinal plant- Lagenaria siceraria. Asian J Pharm Technol. (2019) 9:75–8. doi: 10.5958/2231-5713.2019.00013.8
18. Akhtar MS, Riffat S. Evaluation of anticestodal activity of Lagenaria siceraria (Kaddoo) seeds in sheep. Pakistan Vet J. (1987) 7:139–41.
19. Verma AK, Sharma BD, Banerjee R. Quality characteristics of low-fat chicken nuggets: effect of common salt replacement and added bottle gourd (Lagenaria siceraria L.). J Sci Food Agric. (2012) 92:1848–54. doi: 10.1002/jsfa.5691
20. Sharma SK, Puri R, Jain A, Sharma MP, Sharma A, Bohra S, et al. Assessment of effects on health due to consumption of bitter bottle gourd (Lagenaria siceraria) juice. Indian J Med Res. (2012) 135:49–55. doi: 10.4103/0971-5916.93424
21. Puri R, Sud R, Khaliq A, Kumar M, Jain S. Gastrointestinal toxicity due to bitter bottle gourd (Lagenaria siceraria)– a report of 15 cases. Indian J Gastroenterol. (2011) 30:233–6. doi: 10.1007/s12664-011-0110-z
22. Ho CH, Ho MG, Ho S-P, Ho HH. Bitter bottle gourd (Lagenaria siceraria) toxicity. J Emerg Med. (2014) 46:772–5. doi: 10.1016/j.jemermed.2013.08.106
23. Prajapati R, Umbarkar R, Parmar S, Sheth N. Antidepressant like activity of Lagenaria siceraria (Molina) Standley fruits by evaluation of the forced swim behavior in rats. Int J Nutr Pharmacol Neurol Dis. (2011) 1:152–6. doi: 10.4103/2231-0738.84206
24. Shah BN, Seth AK, Desai RV. Phytopharmacological profile of Lagenaria siceraria: a review. Asian J Plant Sci. (2010) 9:152–7. doi: 10.3923/ajps.2010.152.157
25. Ogunbusola EM. Nutritional and antinutritional composition of calabash and bottle gourd seed flours (var Lagenaria siceraria). J Culin Sci Technol. (2018) 16:326–35. doi: 10.1080/15428052.2017.1390518
26. Ojiako OA, Igwe CU. Nutritional and anti-nutritional compositions of Cleome rutidosperma, Lagenaria siceraria, and Cucurbita maxima seeds from Nigeria. J Med Food. (2007) 10:735–8. doi: 10.1089/jmf.2007.625
27. United States Department of Agriculture (USDA) 2003 Agriculture Research Services (ARS), Nutrient Data Laboratory, National Nutrient Database for Standard Reference, Release # 15. Available online at: http://www.nal.usda.gov/fnic/foodcomp (accessed April 25, 2003).
28. Hassan LG, Sani NA, Dangoggo SM, Ladan MJ. Nutritional value of bottle gourd (Lagenaria siceraria) seeds. Glob J Pure Appl Sci. (2008) 14:301–6. doi: 10.4314/gjpas.v14i3.16812
29. Juee LYM, Naqishbandi AM. Calabash (Lagenaria siceraria) potency to ameliorate hyperglycemia and oxidative stress in diabetes. J Funct Foods. (2020) 66:103821. doi: 10.1016/j.jff.2020.103821
30. Ahmed D, Ashiq N. In vitro analysis of anti-diabetic and anti-oxidative potential of pedicles of fruit-vegetable bottle gourd. Pak J Pharm Sci. (2018) 31:2497–501.
31. Saha P, Mazumder UK, Haldar PK, Gupta M, Sen SK, Islam A. Antioxidant and hepatoprotective activity of Lagenaria siceraria aerial parts. Pharmacogn J. (2011) 3:67–74. doi: 10.5530/pj.2011.23.10
32. Mohan R, Birari R, Karmase A, Jagtap S, Bhutani KK. Antioxidant activity of a new phenolic glycoside from Lagenaria siceraria Stand. fruits. Food Chem. (2012) 132:244–51. doi: 10.1016/j.foodchem.2011.10.063
33. Vijayakumar M, Selvi M, Krishnakumari S. Cardioprotective effect of Lagenaria siceraria (Mol) on antioxidant tissue defense system against isoproterenol-induced myocardial infarction in rats. Inven Impact Ethnopharmacol. (2010) 1:207–10. Available online at: https://d1wqtxts1xzle7.cloudfront.net/30376730/Paper_6-libre.pdf?1390885928=&response-content-disposition=inline%3B+filename%3DCardioprotective_Effect_of_Lagenaria_sic.pdf&Expires=1661937652&Signature=LVl-MHDZj-lCgMTUo5iJfE-evx4trfuhd0t0NijzzPhrfKjeMFrSE0O6XNN4BIGXkPqVZQP0M2PV3pvZ5B1Un8PdNBW9Skwirs3VT0uGMMNUc9rLridI3XGQ8W2PXZWw0oRFUBskdXhCKVXonAmuP3-KSkJ-Eh0dhIG-D9WCZLio0Yz5aq2mOM2pdbDwuop6IrF6k87YY-NqC7IZfrJrCKmNnEayV3wIezFWcTHJPzfVqpqq0RP2l1i7D11kB2xil620NRcUjmJtaA7eKhQxjOQhJqNV2UeKpyhQUcW4oBxO2nc4L3f3-ztD3LbMLhhAbvJ3X3HjchJ~b-LQ_&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
34. Attar UA, Ghane SG. In vitro antioxidant, antidiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.). South Afr J Bot. (2019) 125:360–70. doi: 10.1016/j.sajb.2019.08.004
35. Singh PSB, Prasad G. Lipid-lowering and antioxidant functions of bottle gourd (Lagenaria siceraria) extract in human dyslipidemia. J Evid Based Compl Alt Medi. (2014) 19:112–8. doi: 10.1177/2156587214524229
36. Saha P, Sen SK, Bala A, Mazumder UK, Haldar PK. Evaluation of anticancer activity of Lagenaria siceraria aerial. Int J Cancer Res. (2011) 7:244–53. doi: 10.3923/ijcr.2011.244.253
37. Thakkar JH. Evaluation of in vitro antimutagenic potential of Lagenaria siceraria using Ame's test. Am J Cancer Biol. (2013) 1:57–61. Available online at: https://www.semanticscholar.org/paper/Evaluation-of-invitro-Antimutagenic-Potential-of-Thakkar-PatelChirag/483a97f237ca0414c184333c03a8a45a3043f9f6
38. Kumar N, Kale RK, Tiku AB. Chemopreventive effect of Lagenaria siceraria in two stages DMBA plus croton oil induced skin papillomagenesis. Nutr Cancer. (2013) 65:991–1001. doi: 10.1080/01635581.2013.814800
39. Agrawal RC, Mishra S. Identification and estimation of phytochemicals and evaluation of anticancer activity of Lagenaria siceraria leaves and fruit extract. Int J Curren Key words. (2016) 8:38899–904. Available online at: https://www.journalcra.com/sites/default/files/issue-pdf/17373.pdf
40. Shaneh M. Changes in lung cancer cell line affected by cytotoxicity of Lagenaria siceraria plant extract. Pakistan J Med Health Sci. (2021) 15:1282–4. doi: 10.53350/pjmhs211551282
41. Vigneshwaran V, Thirusangu P, Madhusudana S, Krishna V, Pramod SN, Prabhakar BT. The latex sap of the ‘Old World Plant’ Lagenaria siceraria with potent lectin activity mitigates neoplastic malignancy targeting neovasculature and cell death. Int Immunopharmacol. (2016) 39:158–71. doi: 10.1016/j.intimp.2016.07.024
42. Maqsood M, Ahmed D, Atique I, Malik W. Lipase inhibitory activity of Lagenaria siceraria fruit as a strategy to treat obesity. Asian Pac J Trop Med. (2017) 10:305–10. doi: 10.1016/j.apjtm.2017.03.010
43. Nadeem S. Synergistic effect of Commiphora mukul (gum resin) and Lagenaria siceraria (fruit) extracts in high fat diet induced obese rats. Asian Pacific J Trop Dis. (2012) 2:S883–6. doi: 10.1016/S2222-1808(12)60285-0
44. Erasto P, Mbwambo ZH. Antioxidant activity and HPTLC profile of Lagenaria siceraria fruits. Tanzan J Health Res. (2009) 11:79–83. doi: 10.4314/thrb.v11i2.45206
45. Rai PD, Dambal AA, Khatri S, Khadabadi SS. Development of an anti-obesity polyherbal formulation containing Terminalia arjuna, Lagenaria siceraria and Piper nigrum. Pharma Innov. (2015) 3(11 PartA):33–7. Available online at: https://www.thepharmajournal.com/archives/?year=2015&vol=3&issue=11&ArticleId=473
46. Ghule BV, Ghante MH, Saoji AN, Yeole PG. Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian J Exp Biol. (2006) 44:905–9.
47. Ghule BV, Ghante MH, Saoji AN, Yeole PG. Antihyperlipidemic effect of the methanolic extract from Lagenaria siceraria Stand. fruit in hyperlipidemic rats. J Ethnopharmacol. (2009) 124:333–7. doi: 10.1016/j.jep.2009.04.040
48. Vaibhav A, Singh JP, Singh OP. Role of Lagenaria siceraria fruit juice in overweight and obesity. Int J Res Rev. (2016) 3:6–11. Available online at: https://www.ijrrjournal.com/IJRR_Vol.3_Issue.7_July2016/IJRR002.pdf
49. Gangwal A, Parmar SK, Gupta GL, Rana AC, Sheth NR. Immunomodulatory effects of Lagenaria siceraria fruits in rats. Pharmacogn Mag. (2008) 4:234–8. Available online at: https://eurekamag.com/research/031/822/031822521.php
50. Saha P, Mazumder UK, Haldar PK, Sen SK, Naskar S. Antihyperglycemic activity of Lagenaria siceraria aerial parts on streptozotocin induced diabetes in rats. Diabetol Croat. (2011) 40:49–60. Available online at: https://go.gale.com/ps/i.do?p=AONE&u=googlescholar&id=GALE|A272246332&v=2.1&it=r&sid=AONE&asid=742a7907
51. Bhattacharya S, Das B. Anti-diabetic activity of Lagenaria siceraria pulp and seed extract in normal and alloxan-induced diabetic rats. Int J Pharm Sci Res. (2012) 3:3362–9. Available online at: https://ijpsr.com/bft-article/anti-diabetic-activity-of-lagenaria-siceraria-pulp-and-seed-extract-in-normal-and-alloxan-induced-diabetic-rats/
52. Teugwa CM, Boudjeko T, Tchinda BT, Mejiato PC, Zofou D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in Africa. BMC Complement Altern Med. (2013) 13:1–8. doi: 10.1186/1472-6882-13-63
53. Randive DS, Bhutkar MA, Bhinge SD, Shejawal KP, Sanap PR, Patil PD, et al. Hypoglycemic effects of Lagenaria siceraria, Cynodon dactylon and Stevia rebaudiana extracts. J Herbmed Pharmacol. (2019) 8:51–5. doi: 10.15171/jhp.2019.09
54. Fard MH, Bodhankar SL, Dikshit M. Cardioprotective activity of fruit of Lagenaria siceraria (Molina) Standley on Doxorubicin induced cardiotoxicity in rats. Int J Pharmacol. (2008) 4:466–71. doi: 10.3923/ijp.2008.466.471
55. Mali VR, Mohan V, Bodhankar SL. Antihypertensive and cardioprotective effects of the Lagenaria siceraria fruit in N G-nitro-L-arginine methyl ester (L-NAME) induced hypertensive rats. Pharm Biol. (2012) 50:1428–35. doi: 10.3109/13880209.2012.684064
56. Fard MH, Naseh G, Bodhankar SL, Dikshit M. Cardioprotective effect of Lagenaria siceraria (Molina) Standley (Cucurbitaceae) fruit juice on doxorubicin induced cardiotoxicity in rats. Am J Pharmacol Toxicol. (2010) 5:103–8. doi: 10.3844/ajptsp.2010.103.108
57. Upaganlawar A, Balaraman R. Cardioprotective effects of Lagenaria siceraria fruit juice on isoproterenol-induced myocardial infarction in Wistar rats: a biochemical and histoarchitecture study. J Young Pharm. (2011) 3:297–303. doi: 10.4103/0975-1483.90241
58. Mali VR, Bodhankar SL. Cardioprotective effect of Lagenaria siceraria (LS) fruit powder in isoprenalin-induced cardiotoxicity in rats. Eur J Integr Med. (2010) 2:143–9. doi: 10.1016/j.eujim.2010.03.003
59. Srivastava V, Gupta P, Sharma D. Evaluation of anti-ulcer activity of methanolic extract of Lagenaria siceraria. J Appl Pharm Sci Res. (2021) 4:15–20. doi: 10.31069/japsr.v4i2.4
60. Manchala P. Evaluation of anti-ulcer activity of Lagenaria siceraria chloroform extracts in pylorus ligated rats. Electron J Biol. (2019) 15:27–37. Available online at: https://ejbio.imedpub.com/evaluation-of-antiulcer-activity-of-lagenaria-sicerariachloroform-extracts-in-pylorus-ligated-rats.php?aid=24221
61. Funde SK, Jaju JB, Dharmadhikari SC, Pawar GR. Effect of Lagenaria siceraria fruit extract (Bottle gourd) on hepatotoxicity induced by antitubercular drugs in albino rats. Int J Basic Clin Pharmacol. (2013) 2:728–34. doi: 10.5455/2319-2003.ijbcp20131211
62. Owais F, Mehjabeen. Hepatoprotective effect of Lagenaria siceraria (Linn) in carbamazepine induced hepatotoxicity in rabbits. ISRA Med J. (2019) 10:345–8. Available online at: https://www.researchgate.net/publication/336363154_Hepatoprotective_Effect_of_Lagenaria_Siceraria_Linn_in_Carbamazepine_Induced_Hepatotoxicity_in_Rabbits
63. Lakshmi BVS, Sudhakar M. Antistress activity of Lagenaria siceraria fruit extracts in different experimental models. J Pharm Res. (2011) 4:1013–5. Available online at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.735.8343&rep=rep1&type=pdf
64. Ahmad I, Irshad M, Rizvi MMA. Nutritional and medicinal potential of Lagenaria siceraria. Int J Veg Sci. (2011) 17:157–70. doi: 10.1080/19315260.2010.526173
65. Nagaraja YP, Geetha KN, Vinay MS. Antimicrobial effect of Lagenaria siceraria (Mol.) Standley, against certain bacteria and fungal strains. J Appl Nat Sci. (2011) 3:124–7. doi: 10.31018/jans.v3i1.169
66. Avinash K, Abha D, Ganesh NS. Peptic ulcer: a review with emphasis on plants from Cucurbetaceae family with antiulcer potential. Int J Res Ayurveda Pharma. (2011) 2:1714–6. Available online at: https://www.cabdirect.org/globalhealth/abstract/20123038015
67. Adedapo A, Adewuyi T, Sofidiya M. Phytochemistry, anti-inflammatory and analgesic activities of the aqueous leaf extract of Lagenaria breviflora (Cucurbitaceae) in laboratory animals. Rev Biol Trop. (2013) 61:281–90. doi: 10.15517/rbt.v61i1.11127
68. Palamthodi S, Lele SS. Nutraceutical applications of gourd family vegetables: Benincasa hispida, Lagenaria siceraria and Momordica charantia. Biomed Prev Nutr. (2014) 4:15–21. doi: 10.1016/j.bionut.2013.03.004
69. Shah BN, Seth AK. Screening of Lagenaria siceraria fruits for their analgesic activity. Rom J Biol Plant Biol. (2010) 55:23–6. Available online at: https://www.ibiol.ro/plant/volume%2055/art04.pdf
70. Kalpana VN, Payel C, Rajeswari VD. Lagenaria siceraria aided green synthesis of ZnO NPs: anti-dandruff, anti-microbial anti-arthritic activity. Res J Chem Environ. (2017) 21:14–9. Available online at: https://www.researchgate.net/publication/320878071_Lagenaria_siceraria_aided_green_synthesis_of_ZnO_NPs_Anti-dandruff_Anti-microbial_and_Anti-arthritic_activity
71. Hassan MI, Fouda MA, Hammad KM, Tanani MA, Shehata AZ. Repellent effect of Lagenaria siceraria extracts against Culex pipiens. J Egypt Soc Parasitol. (2014) 44:243–8. doi: 10.21608/jesp.2014.90754
72. Shehata AZI, Mahmoud AM. Efficacy of leaves aqueous extract and synthesized silver nanoparticles using Lagenaria siceraria against Culex pipiens liston and Anopheles pharoensis theobald. J Egypt Soc Parasitol. (2019) 49:381–7. doi: 10.21608/jesp.2019.68148
73. Kalpana VN, Alarjani KM, Rajeswari VD. Enhancing malaria control using Lagenaria siceraria and its mediated zinc oxide nanoparticles against the vector Anopheles stephensi and its parasite Plasmodium falciparum. Sci Rep. (2020) 10:1–12. doi: 10.1038/s41598-020-77854-w
74. Khan MN, Hussain A, Iqbal Z, Sajid MS. Evaluation of anthelmintic activity of Lagenaria siceraria (Molina) Standl and Albizia lebbeck L. against gastrointestinal helminths of sheep. Egypt J Sheep Goats Sci. (2010) 5:1–16. Available online at: https://journals.ekb.eg/article_27383.html
75. Verma A, Jaiswal S. Bottle gourd (Lagenaria siceraria) juice poisoning. World J Emerg Med. (2015) 6:308–9. doi: 10.5847/wjem.j.1920-8642.2015.04.011
76. Miro M. Cucurbitacins and their pharmacological effects. PhytotherRes. (1995) 9:159–68. doi: 10.1002/ptr.2650090302
77. Witkowski A, Knopa J. Binding of the cytotoxic and antitumor triterpenes, cucurbitacins, to glucocorticoid receptors of He La cells. Biochim Biophys Acta. (1981) 674:246–55. doi: 10.1016/0304-4165(81)90382-2
Keywords: Lagenaria siceraria fruit, phytochemistry, pharmacology, nutritional potential, pharmacological effect
Citation: Saeed M, Khan MS, Amir K, Bi JB, Asif M, Madni A, Kamboh AA, Manzoor Z, Younas U and Chao S (2022) Lagenaria siceraria fruit: A review of its phytochemistry, pharmacology, and promising traditional uses. Front. Nutr. 9:927361. doi: 10.3389/fnut.2022.927361
Received: 24 April 2022; Accepted: 25 August 2022;
Published: 16 September 2022.
Edited by:
Gengjun Chen, Kansas State University, United StatesReviewed by:
Syed Nasir Abbas Bukhari, Al Jouf University, Saudi ArabiaSutapa Biswas Majee, NSHM Knowledge Campus, India
Copyright © 2022 Saeed, Khan, Amir, Bi, Asif, Madni, Kamboh, Manzoor, Younas and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sun Chao, sunchao2775@163.com; Muhammad Saeed, msaeed@cuvas.edu.pk
 Muhammad Saeed
Muhammad Saeed Muhammad Sajjad Khan
Muhammad Sajjad Khan Kinza Amir3
Kinza Amir3  Asghar Ali Kamboh
Asghar Ali Kamboh Zahid Manzoor
Zahid Manzoor Sun Chao
Sun Chao