Biology of exploited groupers (Epinephelidae family) around La Réunion Island (Indian Ocean)
- 1Laboratory of Fisheries, Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER) Institute, Boulogne-sur-Mer, France
- 2Délégation Océan Indien, Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER) Institute, Le Port, France
- 3Réserve Naturelle Marine de La Réunion, Saint-Paul, France
The groupers (Epinephelidae family) are demersal species that are a vulnerable resource due to increasing fishing pressure around Reunion Island. Five species of groupers are among the main species exploited by commercial and recreational fisheries around La Réunion Island: blacktip grouper (Epinephelus fasciatus; Forsskål 1775), oblique-banded grouper (Epinephelus radiatus; Day 1868), golden hind (Cephalopholis aurantia, Valenciennes 1828), white-edged lyretail (Variola albimarginata; Baissac 1953) and yellow-edged lyretail (Variola louti; Fabricius 1775). From 2014 to 2021, a total of 482 individuals were caught. Body length-weight relationships showed a significant relationship between total length and total weight for all species. Among the five grouper species, significant sexual dimorphism was only observed for E. fasciatus. For each grouper species, the von Bertalanffy model gave the best fit for the ageing data. While the unconstrained von Bertalanffy model fitted very well to the data of four species (C. aurantia, E. radiatus; V. albimarginata and V. louti), the Gompertz model gave the best fit for the ageing data of E. fasciatus. The parameters of these growth models gave the asymptotic length TL∞ (from 28.9 cm for C. aurantia to 76.6 cm for V. louti), and growth rate K (from 0.16 for V. albimarginata to 0.40 for E. fasciatus) for each species. Consequently the growth performance index for these grouper species varied from 2.40 to 3.09. Based on gonad observation, the length at first sexual maturity of females varied between 14 to 18 cm for C. aurantia, E. fasciatus and V. albimarginata, to 32 cm for E. radiatus and 34 cm for V. louti. The corresponding age at first sexual maturity by species ranged from 1.67 to 6.65 years old. Reproduction intensity showed that reproduction peaked for a period of three months each year. Three species (C. aurantia; E. fasciatus and V. louti) reproduced mainly in summer, between December to March, while E. radiatus and V. albimarginata exhibited peak spawning between April and July. The updated biological parameters for these five species are invaluable inputs into the future assessment and management of these important commercial species.
Introduction
Groupers are a valuable fishery resource of reef ecosystems and are among those species most vulnerable to fishing pressure as they are strongly targeted because of their high commercial value (Heemstra and Randall, 1993). Despite their economic importance, few grouper fisheries are regularly monitored and many are reported to be undergoing declines (Sadovy de Mitcheson et al., 2013; Frisch et al., 2016). The sub-family Epinephelinae (Bleeker, 1874; included in the family Serranidae; Swainson, 1839) comprises 127 valid species (Eschmeyer and Fricke, 2022), which are mostly distributed in tropical and subtropical seas. These groupers are Indo-Pacific taxa known from the east coast of Africa to the western Pacific, including the Red Sea, Japan and Northern Australia, and are patchily distributed throughout their range.
Amongst all species of this Epinephelinae sub-family, the five main species in the commercial catches around La Réunion Island, targeted by artisanal fisheries, are blacktip grouper (Epinephelus fasciatus; Forsskål 1775), oblique-banded grouper (Epinephelus radiatus; Day, 1868), golden hind (Cephalopholis aurantia, Valenciennes, 1828), white-edged lyretail (Variola albimarginata; Baissac 1953), and yellow-edged lyretail (Variola louti; Fabricius 1775) (Biais and Taquet, 1992; Roos et al., 1998; Le Manach et al., 2015). The main demersal tropical fishes, along with snappers (Lutjanidae), groupers (Epinephelidae) and emperors (Lethrinidae), support locally important artisanal fisheries throughout the Indo-Pacific region, but quantitative assessments of these species have been limited by a lack of adequate biological and fisheries data (Newman et al., 2016; Halim et al., 2020), including around La Réunion Island (Le Manach et al., 2015). Since the 2000s, the fisheries area for groupers around La Réunion Island has increased with the popularization of electric fishing reels, employed by both commercial and recreational fishers. Scientific monitoring of La Réunion’s deep demersal species has showed fishing impacts on these resources, with decreased fishing yield and mean landing size from 2000 to 2015 (Roos et al., 2015). There are, however, no management measures or regulations effectively applied on these fisheries, and published data on the grouper species around La Réunion Island are scarce (i.e. growth data for only two species: E. merra (Pothin et al., 2004) and E. radiatus (Mahé et al., 2016)). The aim of the present study is therefore to provide information on the main aspects of growth and reproduction of the five most common grouper species (i.e. new data for Epinephelus fasciatus; Cephalopholis aurantia, Variola albimarginata and Variola louti; and updated growth and new reproduction data for Epinephelus radiatus from Mahé et al., 2016) exploited in the waters of La Réunion Island.
Materials and methods
Sampling
Individuals were sampled during scientific surveys dedicated to these species, and some individuals from commercial landings were added to complete the length range for the adults or the months without surveys around La Réunion Island. From 2014 to 2021, a total of 482 individuals were caught covering the maximum possible number of months specifically to validate the age from marginal increment analysis, and to follow the reproduction periods of these species in the waters around La Reunion Island (the length frequency by species shown in the Supplementary Figure 1). All individuals were taken to the laboratory for accurate measurements. Each individual was measured to the nearest mm for total length (TL) and to the nearest g for total weight (WT). The gonads were observed macroscopically to determine the sex and sexual maturity stage, and were then weighed to evaluate the gonadosomatic index of the females (GSI; i.e. the gonad weight as a proportion of the total gutted weight). Finally, the sagittal otoliths (left and right otoliths) and five dorsal scales taken between the lateral line and the rear of the dorsal fin were collected, cleaned with distilled water and stored dry at room temperature.
Total length/Weight relationship
All pairs of data (total length/total weight) for each species were plotted in order to identify and delete obvious outliers. To estimate the parameters of the allometric L-W relationships, a base-10 logarithm was fitted to data for each species using a linear least squares model:
Where ‘a’ is the intercept and ‘b’ is the slope i.e. the growth coefficient (Le Cren, 1951; Ricker, 1975; Froese, 2006). To investigate variations in the relationship between total length and total weight for each species according to the explanatory variables of sex (S), time, year (Y) and quarter (Q, from Q1 to Q4 for each year), a completed Generalized Linear Model was made. The individual weight was modeled for each species, with body length as a continuous effect and sex and temporal effects as factors, as follows:
With the separate influence of factors sex (Log TL * S), year (Log TL * Y) and quarter (Log TL * Q) on the relationship between body length and weight. When a factor appeared to have a significant effect (P<0.05) on the TL-WT relationship, parameters were estimated for each factor modality.
Ageing procedures
As there has been no previous investigation on the growth of these species, with the exception of E. radiatus, calibration of the ageing method was necessary. Otolithometric and scalimetric approaches were used to determine the age of groupers. Two calcified structures (otoliths and scales) and their associated processing methods (whole scale; whole, burnt and sectioned otolith) were used to ensure the highest resolution pattern of growth rings for analysis. Five scales and two sagittal otoliths were collected for each individual. Several preparation methods of calcified structures (whole scale, whole otolith, burnt whole otolith, sectioned otolith) were tested to obtain the most precise evaluation of the fish age. All calcified structures for each preparation method were photographed using a ZEISS microscope under transmitted light, assisted by an image analysis system using TNPC software for digital processing of calcified structures. The distance between the nucleus and the edge along the longest growth axis was automatically registered using TNPC software. Several measurements were taken by experts for each calibrated image, with the distance between the nucleus and each band considered a growth ring. Finally, in order to limit interpretation error and reading bias, which is defined as the reproducibility of repeated measurements on a given calcified structure, each individual was analysed by two readers and only otoliths with an agreement between readers were used for this study (Chilton and Beamish, 1982). Alternating translucent and opaque bands were visible in the whole otoliths. It was assumed that each annual growth ring consisted of one opaque and one translucent band. The age was therefore expressed in consistent age groups, e.g. a fish in age group 0 has lived between 1 day and 364 days (i.e. between hatching and before 1 year), as recommended by international expert groups (Chilton and Beamish, 1982; Panfili et al., 2002; Vitale et al., 2019). As no previous validation studies (i.e. mark-recapture of wild individuals, captive rearing of either chemically labelled fish of unknown age or of fish of known age, etc.) have been applied to these species, the most widely used indirect validation method, Marginal Increment Analysis (MIA), was used, which assesses the periodicity of increments in calcified structures (Campana, 2001). MIA is a quantitative approach that relies on a measure of the size of the increment under formation (named the marginal increment), i.e., the distance between the most recently formed growth ring and the edge of the otolith, relative to the size of the last fully formed increment. The relative measure of the marginal increment (MI) is given by:
here Ro is the radius of the otolith measured from its focus to the edge, Rn is the distance between the focus and the last growth ring formed n, and Rn-1 is the distance between the focus and the last-but-one growth ring n – 1. If growth rings are formed annually, the marginal increment MI will thus exhibit an intra-annual periodic pattern that can be observed by plotting MI against the date of origin, i.e. the month at which the specimen was captured. MI was measured for each specimen using the otolith (whole otolith for four species and sectioned otolith for E. radiatus) and plotted against the month of capture (the individual numbers per month and per species are shown in Supplementary Table 1). A sinusoidal regression of MI against the month of capture m with a period of 12 months was used to test for the annual periodicity of growth ring formation after linearization:
so that,
with
The global significance of the linear regression provided a statistical validation for an intra-annual pattern in growth ring MI. The classical assumptions of the linear models (normality, homoscedasticity and absence of trends in the residuals) were verified and met.
Growth model estimation
To optimize the growth model with the available data, the length-at-age (TLt) was back-calculated using total length and otolith increment measurements and following the modified Fraser–Lee back-calculation procedure (Campana, 1990) after checking the linear relationship between fish and otolith length:
where TLt is the length-at-age at age t, TLc is total length at capture, TLbi is length at the biological intercept, Rt is otolith radius at age t, Ro is otolith radius at capture, and Rbi is otolith radius at the biological intercept.
Non-linear growth models were fitted to length-at-age data obtained from back-calculated total length. Mean body growth patterns of the fishes sampled were described using four different growth models including:
-the unconstrained Von Bertalanffy (1938) model:
the von Bertalanffy model with forced t0 = 0:
the Gompertz (1825) model:
the logistic model (Verhulst, 1838):
where TL1, TLt, and TL∞ are respectively the length at age 1, at age t and the asymptotic length, and K is the rate at which the asymptote is reached, also called the growth coefficient. The best growth model was identified as the one that minimized the small sample bias-corrected form of the Akaike Information Criterion (AICc; Akaike, 1974; Sakamo et al., 1986). The AICc balances the trade-off between the quality of fit and the number of parameters used (Pauly, 1979) while accounting for small sample bias, and is defined as:
here n is the sample size, k is the total number of parameters of the model and TL is its likelihood.
Fish growth was estimated using the growth performance index (φ) (Pauly and Munro, 1984):
Growth performance index was more appropriate for growth comparison versus comparison of TL∞ and K individually as these two parameters are highly correlated (Sparre et al., 1987). Moreover, the auximetric plot (plane defined by two logarithmic axes representing the von Bertalanffy rate coefficient K versus asymptotic total length TL∞; Boettiger et al., 2012) was used, which allows circumvention of the correlation between TL∞ and K. To compare the growth parameters measured in this study with other studies, von Bertalanffy parameters were extracted for the Serranidae family (n=321 species) from FishBase in May 2022 (Froese and Pauly, 2022).
Finally, the lifespan (tmax) was estimated from the empirical relationship with growth rate K (Froese and Binohlan, 2000) as follows:
any grouper species exhibit protogynous hermaphroditism, changing from females to males at a later size or age (Shapiro, 1987; Pothin et al., 2004), which is also probable for the five studied species and thus the growth of these species was considered without separation by sex.
Sex-ratio and first sexual maturity
Sex was determined by macroscopic observation of the gonads. Sex ratios were calculated as the percentage of females (F) in the samples (F+M: males). To characterize the first sexual maturity, TL50 and tm, were estimated, which represent respectively the average total length at which 50% of individuals in a given population are mature for the first time and the average age at which fish reach sexual maturity. As these species are protogynous hermaphrodites (i.e. change sex from female to male), first sexual maturity was only estimated for females. Age at first sexual maturity was calculated using the inverse of the von Bertalanffy growth function (Froese and Binohlan, 2000) as follows:
Reproduction period
Reproductive maturity stages were assessed macroscopically. Fish were assigned to the following maturity development stages: (I) immature; (II) resting; (III) ripe and running; (IV) spent; and (V) post-spent, as recommended at the international level (ICES, 2018). By monitoring the percentage of individuals within each category for each month throughout the year, particularly for maturity stages III and IV, the reproduction period was identified and the reproduction intensity was quantified according to the monthly gonadosomatic index of the females (GSI).
Statistical analysis
Statistical analyses and plots were performed using the following packages in the statistical environment R: Rfishbase (Boettiger et al., 2012), car (Fox and Weisberg, 2019), ggplot2 (Wickham, 2016) in the R statistical environment (R Core Team, 2021).
Results
Morphological parameters
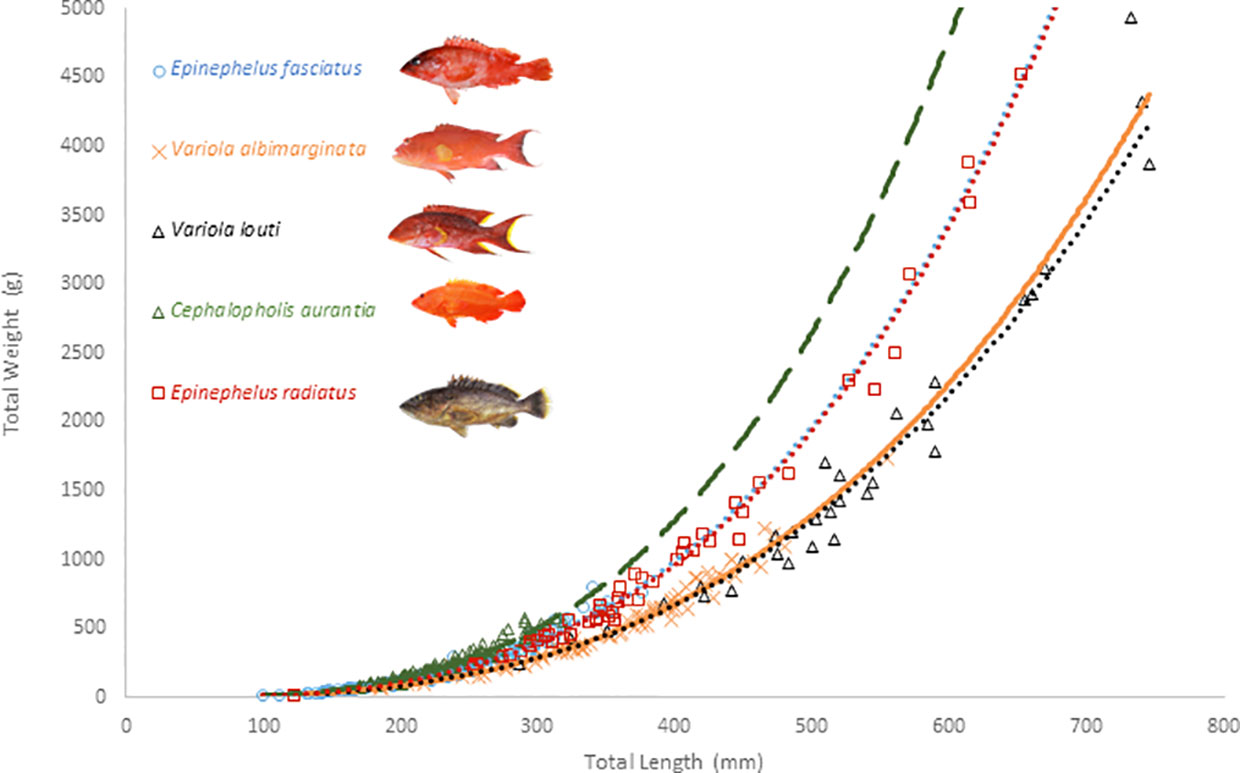
Measured total length and weight for all species ranged from 10.0 cm to 74.6 cm and from 14.7 g to 4940 g respectively, with the lowest values in E. fasciatus and the highest in V. louti (Figure 1, see Table 1 for results by species). Among the five grouper species, all showed a significant correlation (P<0.05) between total length and total weight. The parameters of the body length–weight relationships showed that the initial growth coefficient ‘a’ varied from 3.70·10-6 to 1.46·10-5, while the growth coefficient ‘b’ ranged from 2.95 ± 0.09 for V. louti to 3.29 ± 0.05 for C. aurantia. When b=3.0, the volume of a three-dimensional object is roughly proportional to the cube of length for a regularly shaped solid, which is rarely observed in fish. For V. louti, the specimens have thin, elongated bodies (i.e. b<3.0) while other grouper species have thicker bodies (i.e. b>3.0). The effects of sex and time on the relationship between total length and weight were also tested; no significant difference was found between males and females for any species, nor was there were any significant difference between quarters within each sampling year, for any species (Table 1). Conversely, there was a significant temporal effect on the TL/WT relationship by sampling year for three species (E. fasciatus; E. radiatus; C. aurantia). Among the species, for a given length, the total weight was very similar between the two Epinephelus species and between the Variola species, but C. aurantia had a higher total weight than the other grouper species at the same length.
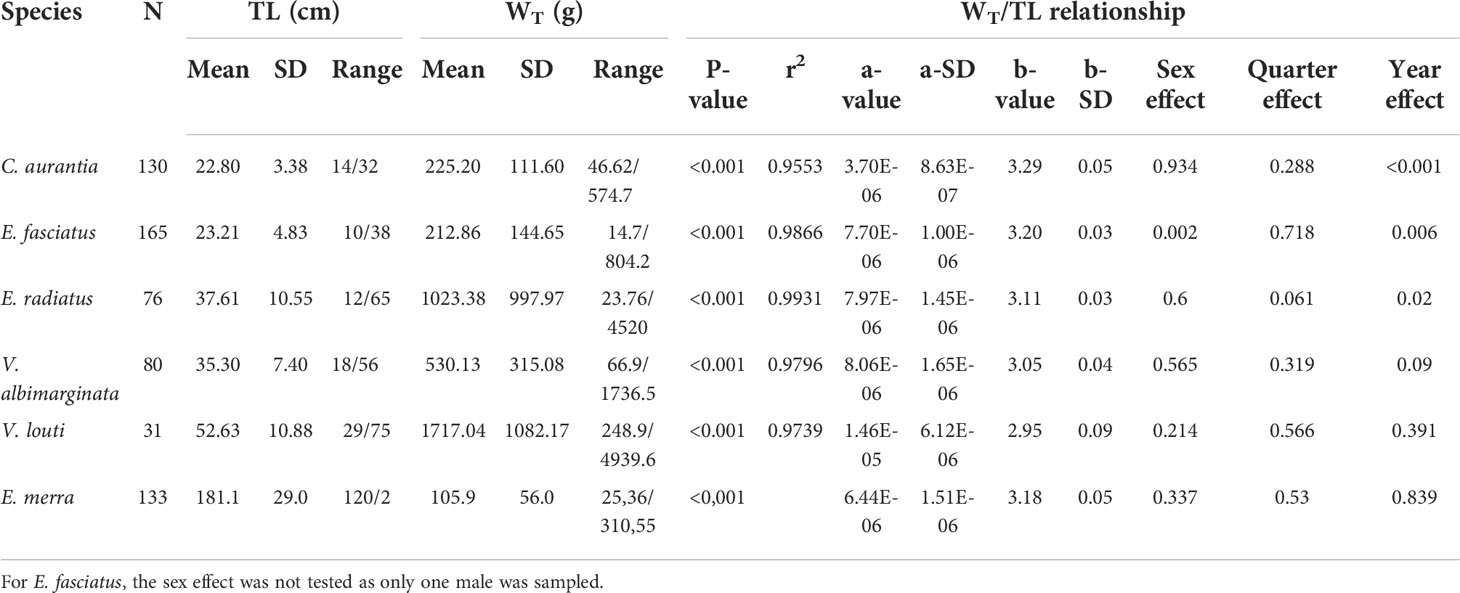
Table 1 Relationships between total length (TL; cm) and total weight (WT; g) (Significance of this relationship, number of individuals, details of each measurement, parameters a and b; P-value of each potential effect).
Growth parameters
Among the five grouper species, laboratory tests were used to evaluate the best calcified structure and its associated preparation technique to estimate fish ageing. The scales were difficult to interpret, while the whole otoliths showed clearer growth structures in all species. Visual comparison confirmed that a fine transverse section presented the same ageing data that the value obtained from the whole otolith, thus reading the whole otolith was the ageing method used in this study (Supplementary Figure 2). To validate the ageing data, the application of MIA to growth increments allowed the detection of significant intra-annual variation in growth (P< 0.05; Figure 2, showing the circannual rhythm of increment formation). The period of maximal marginal increment was not always the same between species. For four species (E. fasciatus, C. aurantia, V. albimarginata and V. louti), the age range was between 0 to 8 years, while older individuals of up to 13 years were observed for E. radiatus (Supplementary Figure 3). The older specimens were males, except in E. radiatus. The predominance of males in older age classes may be indicative of protogynous hermaphroditism. To optimise the statistical precision of the growth model from the individual data (Supplementary Figure 3), the back-calculated length-at-age were used (Figure 3). The unconstrained von Bertalanffy growth model was the best fit for the observed age data from three species (E. radiatus, E. fasciatus and V. albimarginata). Conversely, the von Bertalanffy model with t0 = 0 was the best for the ageing data from two species (C. aurantia and V. louti, Figure 3 and Table 2). The asymptotic length TL∞ varied from 28.90 cm for C. aurantia to 76.57 cm for V. louti (Table 2). The rate at which the asymptotic length was reached, K, was between 0.17 for E. radiatus and 0.40 for E. fasciatus. Consequently, for these grouper species, the growth performance index (φ) varied from 2.40 for C. aurantia to 3.09 for V. louti (Table 2). Comparisons of growth curves of these main grouper species around La Réunion Island showed that growth was rapid during the first four years of life, then slowed with increasing age (Supplementary Figure 4).
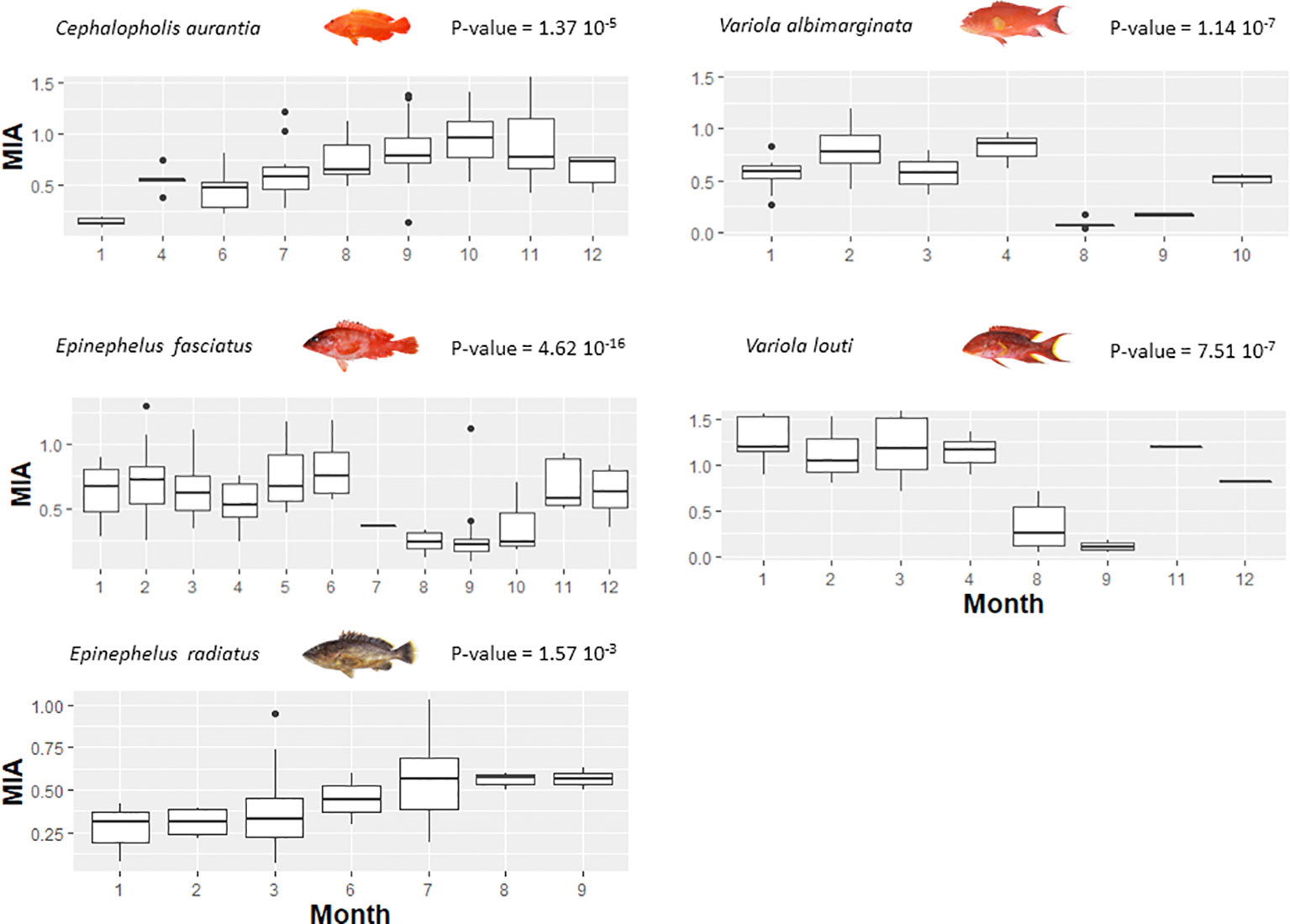
Figure 2 Periodicity validation of growth rings from Marginal Increment Analysis (MIA) of grouper species around La Réunion Island. Under the hypothesis of annual growth rings, an intra-annual periodicity of marginal increment growth is measured by the sinusoidal regression (significant if P-value ≤ 0.05).
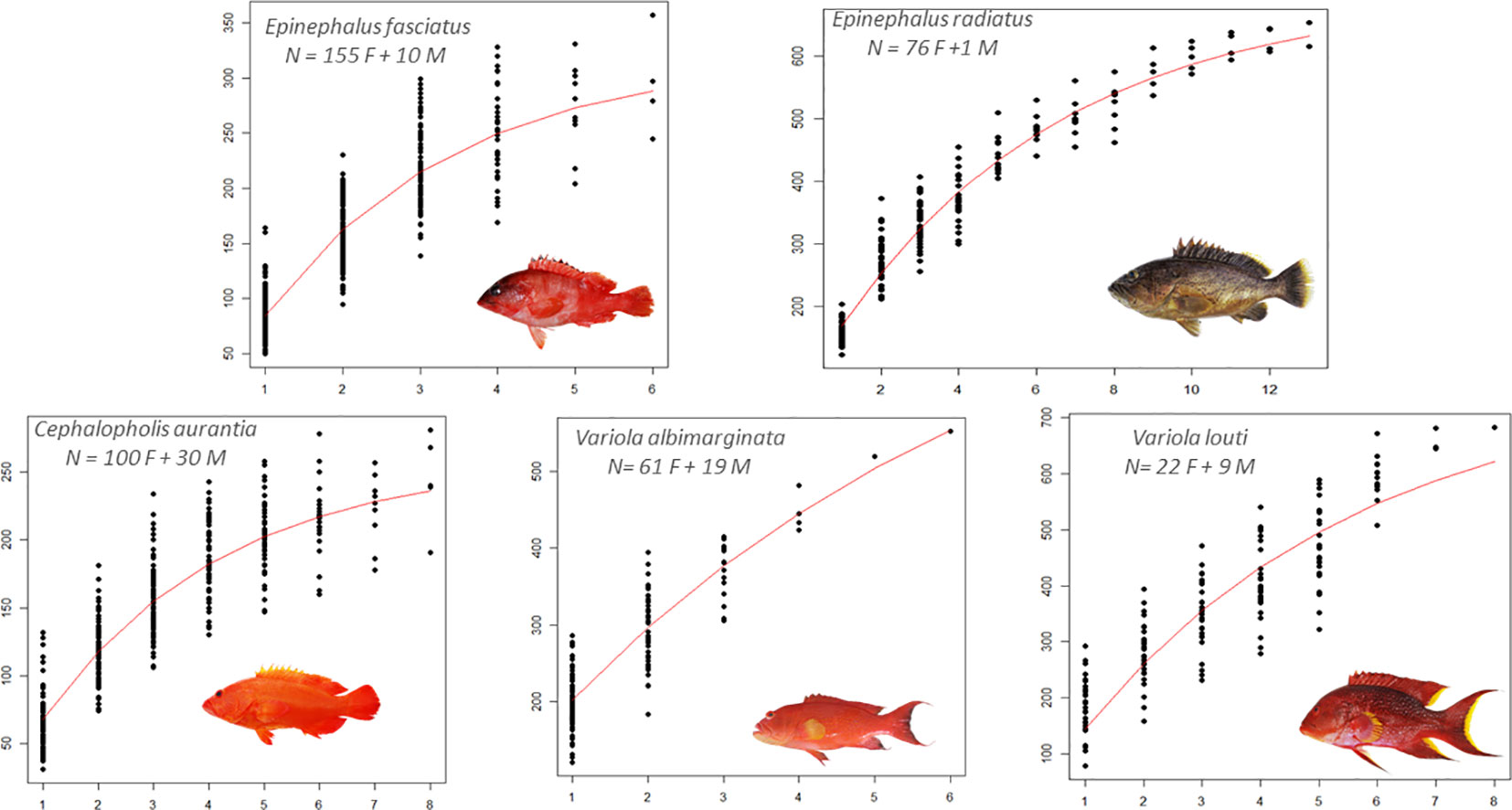
Figure 3 Growth curves of grouper species around La Réunion Island fitted to all individual length-at-age modified back calculation method [The specimen number (N) is represented with the females (F) and the males (M)].
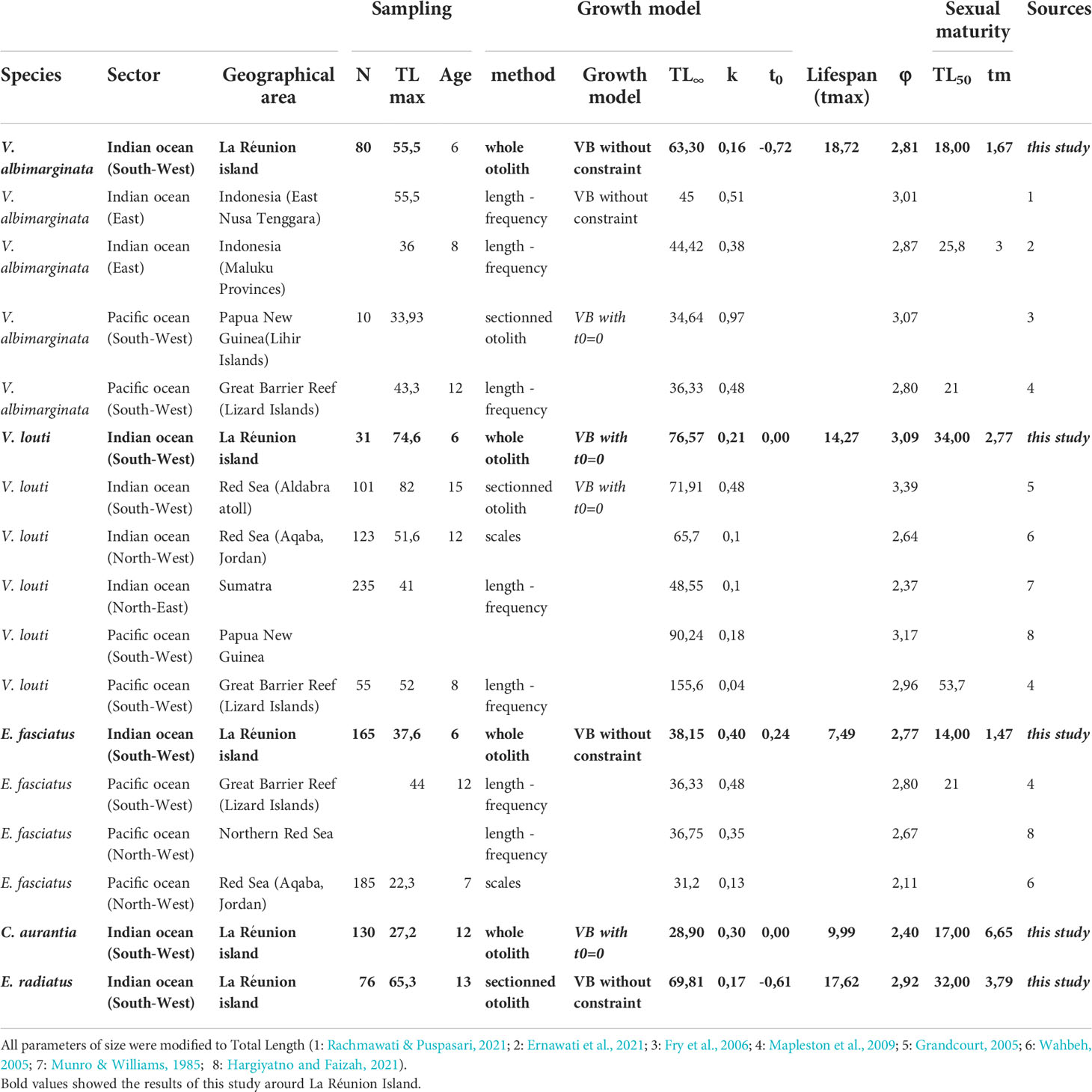
Table 2 Biogeographic comparison of the biological parameters of grouper species with the sampling details (number of samples, observed maximum TL and Age) of the growth model (model used with the type of data and the parameters of the growth model and growth performance index (φ)) and the first sexual maturity (observed TL and Age (tm).
Reproduction parameters
Among 482 individuals caught around La Réunion Island, 413 were females and only 69 were males. Consequently, the overall sex ratio for all grouper species was in favour of females, which represented 85.7% of the individuals (Figure 3). For each species, the sex ratio value estimated by each sampling month was comprised between 50% and 100% (i.e. the monthly number of females was always higher than the males number; Supplementary Table 2). The percentage of females in the sample varied from 70.96% to 98.70% by species, and the mean size of females was significantly smaller than that of males due to the dominance of females in the lower length classes and of males in the higher classes.
The observed length at first sexual maturity of females (i.e. TL50, the average total length at which 50% of individuals in a given population are mature for the first time) showed one group of species (C. aurantia; E. fasciatus and V. albimarginata) with TL50 values between 14 and 18 cm and a second group with TL50 values between 32 cm (E. radiatus) and 34 cm (V. louti) (Table 2). From the growth model parameters, corresponding age of first sexual maturity by species ranged from 1.47 (E. fasciatus) to 6.65 years old (C. aurantia) (Table 2). The monthly sampling throughout the years showed that all five species had reproductively capable specimens present in all months, but peak reproduction occurred during a period of three months (Figure 4). The austral summer (i.e. between December to March) was peak spawning time for three species (C. aurantia; E. fasciatus and V. louti) while the two other species (E. radiatus and V. albimarginata) exhibited peak spawning from April to July (Figure 4).
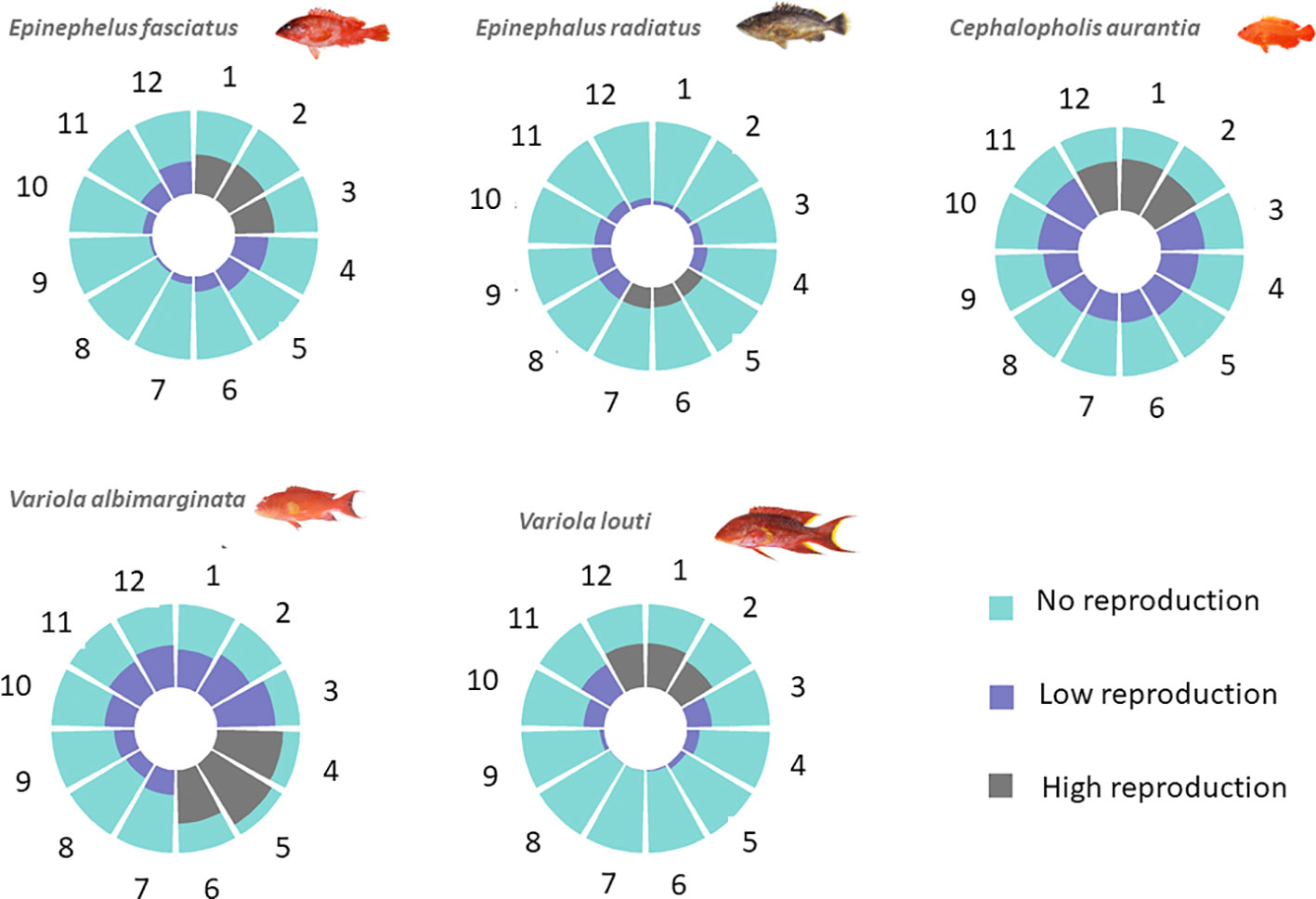
Figure 4 Reproduction period and intensity of grouper species around La Réunion Island. The reproduction intensity was quantified according to the monthly level of the gonadosomatic index (GSI) of the females.
Discussion
Demography and associated habitat
These five species are likely to be monandric protogynous hermaphrodites, which is common in several grouper species (Heemstra and Randall, 1993). This sequential hermaphroditism is reflected in the length ranges of females versus males seen here, where the smaller length classes are dominated by females while the larger length classes are almost exclusively composed of males. For these species, the sex ratio is skewed towards females (Morgans, 1982; Sadovy and Shapiro, 1987; Shapiro, 1987; Molloy et al., 2007; Mapleston et al., 2009; Ohta et al., 2017), as seen here for all species around La Réunion Island (Pothin et al., 2004). For the length classes with both females and males, there was no significant sexual dimorphism in the relationship between total length and weight. The proportion of males in the samples was, however, very low, which may partly explain this result. A significant temporal effect on the TL/WT relationship was observed among sampling years for three species. This trend could be explained by a difference in available food composition and/or quantity between years for the sampled individuals.
To understand the demography (i.e. population size and composition by life stage and sex) and consequently optimize the sampling design, it is important to know the social structure and behaviors, particularly those associated with reproduction or feeding (Liu and Sadovy, 2005; Roos pers. comm., from underwater visual system, Supplementary Video). For smaller groupers, such as the five grouper species in this study, adults are commonly associated with corals reefs or rocky structures and are strongly site-attached (Heemstra and Randall, 1993), generally with a single male and several females. For these protogynous hermaphrodite species, behaviors according to the life stage and sex influence the catchability of different individuals and therefore the representativeness of the sample (Colin and Clavijo, 1988; Donaldson, 1989; Shpigel and Fishelson, 1989; Mackie, 1993; Shapiro et al., 1993; Donaldson, 1995; Sluka and Sullivan, 1996; Sluka, 2001; Liu and Sadovy, 2005). All studied Cephalopholis and several Epinephelus species, for example, live in relatively small and well-defined social groups in the wild, consisting of a single male, and one to several smaller females (Colin et al., 1987; Liu and Sadovy, 2005), although most grouper species are known to be solitary (Heemstra and Randall, 1993). Habitats ranges of individuals are significantly different between males and females (Liu and Sadovy, 2005). While we randomly sampled the specimens during the surveys and from commercial catches, the female-skewed sex ratio observed for all species is strongly linked to the social structure of grouper species. Our sampling of E. radiatus, for example, found only one male for 76 females. We sampled during all quarters for several years from 2004 to 2021, but the individuals may group together during the reproductive season, or, alternatively, avoid each other as males defend their respective territories at different times during the annual cycle, especially during the reproductive season (Morgans, 1982; Liu and Sadovy, 2005; Kline et al., 2011). Consequently, the geographic distribution of males and females varies throughout the year and the period of sampling likely influenced the sex-ratio of grouper species found here. There are also catchability differences between sexually active individuals that move to spawning aggregations, and the small, sexually inactive individuals that are residents, which would explain difficulties in sampling the smallest individuals. For all species, the smallest specimen measured 10 cm (Table 1; E. radiatus). In situ observation from submarine video show territorial behavior where smaller specimens of Variola species were excluded from territories by bigger fish (Roos, pers. comm.). While social structure and associated behavior likely influenced the sampling of groupers, there are additional effects that could also explain the difference in sampling composition. There is an inverse correlation between the level of fishing pressure on a given population, typically concentrated on the larger and older groupers, and the proportion of large individuals and/or the size at first sexual maturity (Coleman et al., 2000; Nash et al., 2016), although our study showed that the larger specimens sampled and the maximum total length observed around La Réunion Island have very similar values to those found in other studies on these species (Table 2). Finally, while the combination of social structure and human activities (i.e. fishing, climate change, disease, pollution, etc.) may have influenced the demography of all groupers, there are other species-specific factors such as relationships between depth range and size range that could also be influential on the size distribution sampled here (Williams, 1991; Sluka and Sullivan, 1996).
Growth
Age structure and growth data, required for stock assessments, were analyzed for these five grouper species around La Réunion Island. Ageing data were estimated from the sagittal otolith, as is standard for many grouper species (Mapleston et al., 2009; Condini et al., 2014). Visual tests showed that the use of the scales was very limited and subject to potential bias in age estimation as the patterns in growth rings were not clearly linked to annual growth. Previously, the scales of E. fasciatus and of V. louti have been used for ageing these species in the Red Sea, but without comparison with the otoliths (Wahbeh, 2005). This difference between geographical areas in effectiveness of the scales for ageing the same species may be related to environmental and ecological differences (e.g. factors such as temperature variations, or other factors as food availability and composition). In contrast, the otoliths presented well-defined growth increments, and were used for all species, but the preparation method was varied among grouper species. For four species, whole otolith reading was the most reliable method to observe the growth increments, but the transversal section showed better precision for the ageing data for E. radiatus. From these otolith preparations, annual periodicity was validated for each species by the marginal increment analysis. This method is indirect and could be confirmed in the future by direct validation methods such as mark-recapture of wild individuals. Two previous studies, however, used sectioned otoliths for both Variola species (V. albimarginata and V. louti), (Grandcourt, 2005; Ernawati et al., 2021). As with the choice of calcified structure, the best preparation method could be linked to the studied geographical area and its intrinsic features, but also to the differences in methods developed between laboratories. The potential bias between the preparation methods, however, is very limited compared to the imprecision that can be observed between two calcified structures (Vitale et al., 2019). For groupers species showing the small sample size, it was not easy to obtain the efficient number of specimen for all length classes. Consequently, the back-calculation method of the length-at-age increase the data number, especially in the first life stages as juveniles) and the statistical precision of the growth model (Wilson et al., 2009). Finally, the choice of growth model is the last source of bias unrelated to the biology of the species for comparing growth results between or within species. Among different growth models (i.e. non-linear mathematical models), the von Bertalanffy model without constraint or with t0 equal to 0 was the best growth model (i.e. gave the smallest value of AICc) that fitted the data for all the grouper species. In the future, the sampling effort of young fish must be carried out to validate the choice of the best model for each species, as these data are important to adjust the different models. The von Bertalanffy model showed the highest accuracy based on the deviation between the individual data field and the model estimation at ages, and its biological interpretation could be different before first sexual maturity (Katsanevakis and Maravelias, 2008). Consequently, when growth is compared between several studies, growth performance index (Supplementary Figure 5) and an auximetric plot (Figure 5) could be added to the growth parameters of each model to limit the difference between the model types. The observed values of growth performance index for the grouper species around La Réunion Island are in accordance with those extracted from the literature (Supplementary Figure 5). Using the growth performance index to compare the global body growth among all grouper species showed that Epinephelus species are among the fastest growing groupers, with similar asymptotic growths (321 species extracted from FishBase). Consequently, for the same environmental conditions, Epinephelus species may be less vulnerable than other groupers to environmental or anthropogenic pressures.
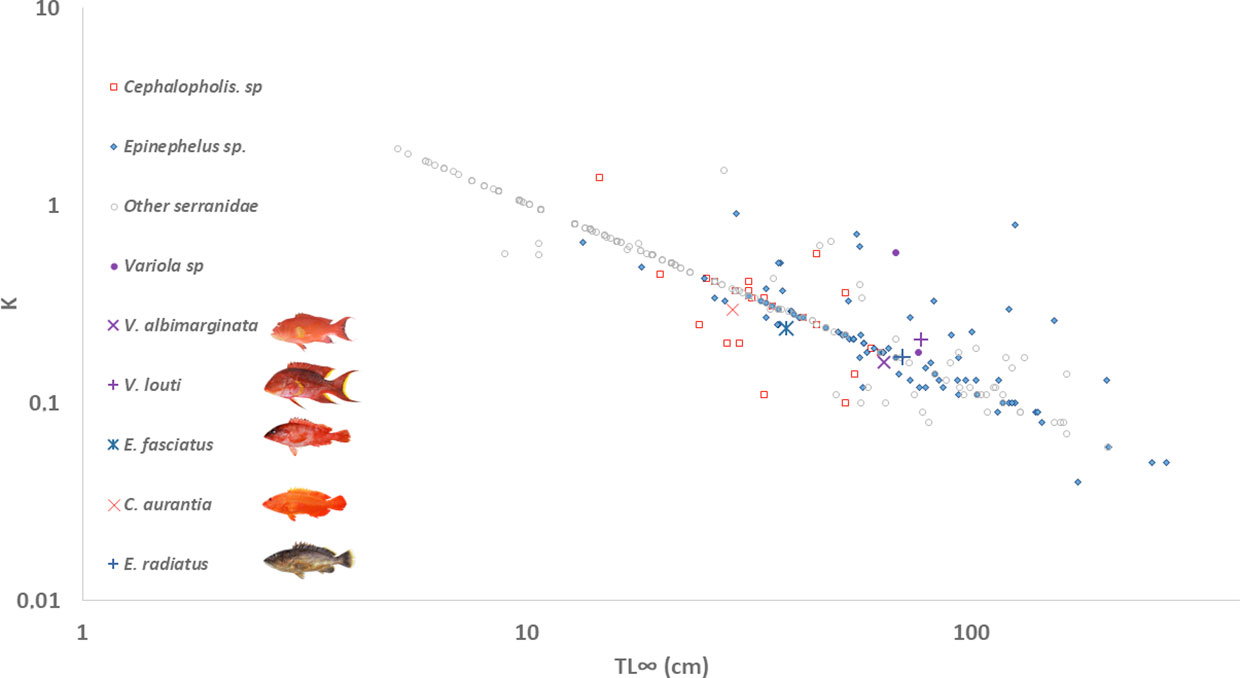
Figure 5 Auximetric plot of five grouper species from La Réunion Island relative to other species of the Serranidae family (Source Fishbase, n=321 species, only the Total Lengths were used). The auximetric plane is the plane defined by two logarithmic axes representing the von Bertalanffy rate coefficient K versus asymptotic total length TL∞, where a population characterized by a set of von Bertalanffy growth parameters (TL∞), is represented by a point.
The growth models among five grouper species around La Réunion Island showed the same patterns, with fast growth during the first years, and a growth rate that decreases substantially between the ages from 4 to 8 years (Supplementary Figure 3). The oldest specimens by species are between 6 (C. aurantia, V. albimarginata and V. louti) and 13 years old (E. radiatus), while the lifespan (tmax) ranged from 8 years (E. fasciatus) to 19 years (C. aurantia) (Table 2). Asymptotic total length observed for the grouper species ranged from 28.9 cm for C. aurantia to 76.26 cm for V. louti (Table 2). These values place these species in the intermediate-sized groupers (i.e. neither dwarf nor giant groupers, Heemstra and Randall, 1993). The growth coefficient (k) ranged from 0.17 to 0.40, showing relatively fast growth for these species (Table 2). While no previous growth study was available for C. aurantia or E. radiatus, it is possible to compare our results to previous studies for the other three species (Table 2). For V. albimarginata, five previous studies were published (Fry et al., 2006; Mapleston et al., 2009; Damora et al., 2021; Ernawati et al., 2021; Rachmawati and Puspasari, 2021); no measured asymptotic total length values exceeded 45 cm, although individuals of up to 55.5 cm were observed in situ. In our study, the maximum total length measured for this species was 55.5 cm, and the asymptotic length of 63.3 cm calculated at La Réunion Island seems to be a good estimation for V. albimarginata; this maximum size is much higher than previously observed (Supplementary Figure 5). This higher growth rate could be due to environmental conditions and/or an indicator of high fishing pressure on this species at La Réunion Island, as observed for other species (Marty et al., 2014). For V. louti, five studies have previously been published (Munro and Williams, 1985; Grandcourt, 2005; Wahbeh, 2005; Mapleston et al., 2009; Hargiyatno and Faizah, 2021). The growth observed at La Réunion Island is very similar to that of Papua New Guinea (Munro and Williams, 1985); the asymptotic total length was calculated here as 76.6 cm, which is close to the maximum total length observed on the Seychelles bank (i.e. 82 cm; Grandcourt, 2005; Table 2). The model growth from the length frequency analysis study by Mapleston et al. (2009) seems to strongly overestimate the growth of this species (particularly with TL∞=155 cm when the in situ data showed TLmax=52 cm). Finally, the growth of E. fasciatus around La Réunion Island corroborated other observed growth models and parameters in the Red Sea (Wahbeh, 2005; Saleh et al., 2019) and in the South-West Pacific Ocean (Mapleston et al., 2009) (Supplementary Figure 6).
Reproductive biology
The first major reproductive biology trait investigated here in grouper species around La Réunion Island is the first sexual maturity from the average age (tm) and length (TL50) at which fish reach sexual maturity. The length at first sexual maturity of females is smaller than the length at sex change (Frisch et al., 2016). First sexual maturity is reached between 14 and 18 cm in the three smallest species (V. albimarginata; E. fasciatus and C. aurantia), and between 32 and 34 cm in the two larger species (E. radiatus and V. louti) (Table 2). These values are smaller than those observed in other geographical areas in the East Indian Ocean (Indonesia) and in the South-West Pacific Ocean (Great Barrier Reef) for the same species (V. albimarginata: Mapleston et al., 2009; Ernawati et al., 2021, V. louti: Mapleston et al., 2009; E. fasciatus: Mapleston et al., 2009). These differences, particularly reduction of the length at the sexual maturity, can be caused by phenotypic changes, genetic adaptations or both (Law, 2000). The length at maturity is influenced by environmental conditions (i.e. temperature, food resources, etc.) (Weatherley, 1990), but other potential factors such as human activities, particularly fishing, could result in such diminution. A negative relationship between the length at sexual maturity and the level of fishing pressure is among the indicators of fishery-induced changes in stocks (ICES, 2012; Marty et al., 2014). The low value of TL50 observed around La Réunion Island could consequently be linked to high fishing pressure on these species. Finally, social and size structure, important features for grouper species, could also modify this life history trait (Hutchings et al., 1999). From TL50, the age at first sexual maturity was estimated between 1.5 (E. fasciatus) and 6.7 years (C. aurantia) for these grouper species (Table 2). Only one recent study, on V. albimarginata in Indonesia, estimated this parameter for this species and showed a higher age at maturation than was found in the present study (Ernawati et al., 2021).
The second major reproductive biology trait investigated here was the timing and intensity of the spawning period. Individuals are able to reproduce almost all year round, however, by identifying the reproduction intensity according to the monthly level of the gonadosomatic index (GSI), it is possible to define the optimal spawning period precisely. For all five grouper species, the duration of this period was found to be between 2 and 3 months (Figure 4), which corroborated previously observed results from other grouper species (Nemeth et al., 2007; Robinson et al., 2008). This reproduction peak is not, however, at the same time of year among different groupers in the same geographical area. Additionally, this period can be different between different geographical areas within the same species, as for three of the species studied here (E. fasciatus; V. albimarginata; V. louti), which showed different timing between La Réunion Island and the Great Barrier Reef (Mapleston et al., 2009), or for other grouper species, such as Epinephelus polyphekadion (Ohta et al., 2017). Lunar periodicity seems to be the most influential external stimulus on reproductive characteristics, particularly spawning time, of fish species inhabiting shallow waters in tropical and subtropical zones (Harrison et al., 1984; Thresher, 1984); the honeycomb grouper Epinephelus merra, for example, is a typical lunar spawner with full moon preference (Fukunaga et al., 2020). One possible reason why such patterns are typical of these regions is that the lower variation in water temperature and photoperiod in these zones is correlated with a relative increase in the importance and reliability of cues from the moon (Takemura et al., 2010).
Implications for life history and conservation
Among grouper species, there are three groups that show strongly divergent life history traits (Heemstra and Randall, 1993). Firstly, there are the giant species of groupers (i.e. species that exceed 2 m in total length, including E. lanceolatus, E. itajara, E. quinquefasciatus, Hyporthodus nigritus), which have relatively slow growth, aggregation spawning, low instantaneous rates of natural mortality and long life-spans (Heemstra and Randall, 1993); these species should be considered k-strategists (Huntsman et al., 1999). Their distribution patterns show high abundance of smaller individuals in inshore waters and low abundance of larger individuals in offshore waters (Sluka and Sullivan, 1996). Secondly, there are the dwarf species of groupers as “showing opposite life history and distribution patterns to the giant species (i.e. r-strategists) (Sluka and Sullivan, 1996). Thirdly, there are intermediate grouper species that are neither dwarf nor giant groupers, such as the species around La Réunion Island. According to the ecological characteristics of each species, the patterns of distribution and consequently the fishing effort, typically concentrated on the large specimens, are very different (Coleman et al., 2000). The position of five grouper species around La Réunion Island in the auximetric plot for Serranidae species showed that they are part of the average range and neither giant nor dwarf species (Figure 5). The lifespan of these five species in La Réunion Island waters was estimated between 8 (E. fasciatus) and 19 years (C. aurantia) (Table 2); these values are very far from the 61 years observed for E. marginatus in the Indian Ocean (Fennessy, 2006). The ratio between the life span and the age of sexual maturity, however, indicated ecological characteristics close to those of the dwarf species, more akin to r-strategists (short lived, reproduce at an early age, fast sexual maturity).
In this study, we provide novel data on life history and reproductive biology parameters for five of the most highly exploited grouper species around La Réunion Island. These data are essential for effective stock assessment and management of these fishes under scenarios of both changing environmental conditions and increasing fishery exploitation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: SEANOE - Oceanographic Data.
Author contributions
DR and KéM designed the research. CG, BB, HE, CL, RB-M, and TR realized the sampling and estimated the sexual parameters. CG, BB, and HE carried out the otolith extraction and the images, ST, AD, and RE realized the ageing data. KéM, KiM, and D.R. analyzed all data and performed the statistical analyses. All authors provided input for the results and discussion. KéM, KiM, and DR wrote the paper. All authors provided critical comments and were involved in the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was carried out with the financial support of the Data Collection Framework (DCF; EC Reg. 199/2008, 665/2008; Decisions 2008/949/EC and 2010/93/EU), the European Fisheries Fund (EFF 2007-2013; ANCRE-DMX2 project : Indicateurs biologiques et écologiques pour une gestion durable des stocks de poissons DéMersauX profonds d’intérêt halieutique à La Réunion), The European Maritime and Fisheries Fund (EMFF 2014-2020; IPERDMX project : Indicateurs Populationnels et Ecosystémiques pour une gestion durable des Ressources en poissons DéMersauX récifaux et profonds (1-500 m) à la Réunion) and the French State. Another project ’PECHTRAD’ (PECHe TRADitionnelle) funded by the reserve participated in this study.
Acknowledgments
We thank all fishers and colleagues who helped us in the field, and the reviewers for their comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor PC declared a past collaboration with the author KM.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.935285/full#supplementary-material
References
Akaike H. (1974). A new look at the statistical model identification. IEEE Trans. Autom. Control. 19, 716–723. doi: 10.1109/TAC.1974.1100705
Biais G., Taquet M. (1992). La pêche locale aux abords de la réunion (Editions de l'Ifremer). Available at: https://archimer.ifremer.fr/doc/00000/1455/.
Bleeker P. (1874). Mémoire sur les Sciénoïdes et les Sillaginoïdes de l’Inde archipélagique. Verslagen en Mededeelingen der Koninklijke Akademie van Wetenschappen. Afdeeling Natuurkunde 14: 1–76.
Boettiger C., Lang D. T., Wainwright. P. C. (2012). Rfishbase: exploring, manipulating and visualizing FishBase data from r. J. Fish Biol. 81 (6), 2030–2039. doi: 10.1111/j.1095-8649.2012.03464.x
Campana S. E. (1990). How reliable are growth back-calculations based on otoliths? Can. J. Fish. Aquat. Sci. 47, 2219–2227. doi: 10.1139/f90-246
Campana S. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59 (2), 197–242. doi: 10.1111/j.1095-8649.2001.tb00127.x
Chilton D. E., Beamish R. J. (1982). Age determination methods for fishes studies by the groundfish program at the pacific biological station. Can. Spec. Publ. Fish. Aquat. Sci. 60, 1–102.
Coleman F., Koenig C., Huntsman G., Musick J., Eklund A., MCGovern J.M., et al. (2000). Long-lived reef fishes: The grouper-snapper complex. Fisheries. 25, 14–21. doi: 10.1577/1548-8446(2000)025%3C0014:LRF%3E2.0.CO;2
Colin P. L., Clavijo I. E. (1988). Spawning activity of fishes producing pelagic eggs on a shelf edge coral reef, southeastern Puerto Rico. Bullet. Mar. Sci. 43, 249–279.
Colin P. L., Shapiro D. Y., Weiler D. (1987). Aspects of the reproduction of two species of groupers, epinephelus guttatus andE. striatusin theWest indies. Bullet. Mar. Sci. 40, 220–230.
Condini M. V., Albuquerque C. Q., Garcia A. M. (2014). Age and growth of dusky grouper (Epinephelus marginatus) (Perciformes: Epinephelidae) in the southwestern Atlantic, with a size comparison of offshore and littoral habitats. Fish. Bull. 112 (4), 311–321. doi: 10.7755/FB.112.4.7
Damora A., Fadli N., Muchlisin Z. A., Dewiyanti I., Batubara A. S., Nur F. M., et al. (2021). White-edged lyretail (Variola albimarginata): A preliminary. IOP Conf. Ser.: Earth Environ. Sci. 674, 12091. doi: 10.1088/1755-1315/674/1/012091
Donaldson T. J. (1989). Pair spawning of cephalopholis boenak (Serranidae). Jpn. J. Ichthyol. 35, 497–500. doi: 10.1007/BF02905511
Donaldson T. J. (1995). ). courtship and spawning behavior of the pygmy grouper, cephalopholis spiloparea (Serranidae: Epinephelinae), with notes on c. argus and c. urodeta. Environ. Biol. Fish. 43, 363–370. doi: 10.1007/BF00001171
Ernawati T., Agustina A., Kembaren D. D., Yulianto I., Satria F. (2021). Life history parameters and spawning potential ratio of some reef fish species in fisheries management area 715 of Indonesia. AACL Bioflux 14 (5), 3092–3103.
Eschmeyer W. N., Fricke R. (2022) Catalog of fishes electronic version. Available at: https://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp.
Fennessy S. T. (2006). Reproductive biology and growth of the yellowbelly rockcod epinephelus marginatus (Serranidae) from south-East Africa. Afr. J. Mar. Sci. 28, 1–11. doi: 10.2989/18142320609504128
Fox J., Weisberg S. (2019) (Thousand Oaks CA: Sage). Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Frisch A. J., Cameron D. S., Pratchett M. S., Williamson D. H., Williams A. S., Reynolds A. D., et al. (2016). Key aspects of the biology, fisheries and management of coral grouper. Rev. Fish Biol. Fisheries 26, 303–325. doi: 10.1007/s11160-016-9427-0
Froese R. (2006). Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichth. 22 (4), 241–253. doi: 10.1111/j.1439-0426.2006.00805.x
Froese R., Binohlan C. (2000). Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 56 (4), 758–773. doi: 10.1111/j.1095-8649.2000.tb00870.x
Froese R., Pauly D. (2022). FishBase (World Wide Web electronic publication). Available at: www.fishbase.org.
Fry G. C., Brewer D. T., Venables W. N. (2006). Vulnerability of deepwater demersal fishes to commercial fishing: Evidence from a study around a tropical volcanic seamount in Papua new Guinea. Fish. Res. 81, 126–141. doi: 10.1016/j.fishres.2006.08.002
Fukunaga K., Yamashina F., Takeuchi Y., Yamauchi C., Takemura A. (2020). Moonlight is a key entrainer of lunar clock in the brain of the tropical grouper with full moon preference. BMC Zool. 5, 1–11. doi: 10.1186/s40850-020-00060-8
Gompertz B. (1825). On the nature of the function expressive of the law of human mortality and on a new mode of determining the value of life contingencies Vol. 115 (Philos. Trans. R. Soc. Lond), 515–585. doi: 10.1098/rspl.1815.0271
Grandcourt E. (2005). Demographic characteristics of selectedepinepheline groupers (Family: Serranidae; Subfamily:Epinephelinae) from aldabra atoll, Seychelles. Atoll Res. Bull. 539, 201–210. doi: 10.5479/si.00775630.539.199
Halim A., Loneragan N. R., Wiryawan B., Hordyk A. R., Sondita M. F. A., Yulianto I. (2020). Evaluating data-limited fisheries for grouper (Serranidae) and snapper (Lutjanidae) in the Coral Triangle, Eastern Indonesia. Reg. Stud. Mar. Sci. 38, 101388.
Hargiyatno I. T., Faizha R. (2021). Population parameters of the yellow-edged lyretail (Variola louti, forsskål 1775) in sibolga waters. E3S Web Conferences 322, 1015. doi: 10.1051/e3sconf/202132201015
Harrison P. L., Babcock R. C., Bull G. D., Oliver J. K., Wallace C. C., Willis B. L. (1984). Mass spawning in tropical reef corals. Sci. 223, 1186–1189. doi: 10.1126/science.223.4641.1186
Heemstra P. C., Randall J. E. (1993). FAO species catalogue. vol. 16. groupers of the world (family serranidae, subfamily epinephelinae). an annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper and lyretail species known to date. Rome: FAO. FAO Fish. Synop. 125 (16), 382. doi: 10.1016/j.rsma.2020.101388
Huntsman G. R., Potts J., Mays R. W., Vaughan D. (1999). “Groupers (Serranidae, epinephelinae): endangered apex redators of reef communities,” in Life in the slow lane: ecology and conservation of long-lived marine animals. Ed. Musick J. A. (Bethesda: American Fisheries Society), 217–231.
Hutchings J. A., Bishop T. D., McGregor-Shaw C. R. (1999). Spawning behaviour of Atlantic cod, gadus morhua:evidence of mate competition and mate choice in a broadcast spawner. Can. J. Fish. Aquat. Sci. 56, 97–104. doi: 10.1139/f98-216
ICES (2012). Marine strategy framework directive - descriptor 3+. ICES CM 2012/ACOM:62. (Copenhaguen, Denmark: ICES) 172.
ICES (2018). Report of the workshop for advancing sexual maturity staging in fish (WKASMSF). ICES CM/EOSG: 38 (Copenhaguen, Denmark: ICES). 75.
Katsanevakis S., Maravelias C. D. (2008). Modelling fish growth: multi-model inference as a better alternative to a priori using von bertalanffy equation. Fish Fish. 9, 178–187. doi: 10.1111/j.1467-2979.2008.00279.x
Kline R. J., Khan I. A., Holt G. J. (2011). Behavior, color change and time for sexual inversion in the protogynous grouper (Epinephelus adscensionis). PloS One 6 (5), e19576. doi: 10.1371/journal.pone.0019576
Law R. (2000). Fishing, selection and, phenotypic evolution, ICES. J. Mar. Sci. 57, 659–668. doi: 10.1006/jmsc.2000.0731
Le Cren E. D. (1951). The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219. doi: 10.2307/1540
Le Manach F., Bach P., Barret L., Guyomard D., Fleury P. G., Sabarros P. S., et al. (2015). “Reconstruction of the domestic and distant water fisheries catch of la réunion (France), 1950–2010,” in Fisheries catch reconstructions in the Western Indian ocean 1950–2010, vol. 23 . Eds. Manach F., Pauly D. (Fisheries Centre, University of British Columbia: Fisheries Centre Research Reports), 83–98.
Liu M., Sadovy Y. (2005). Habitat association and social structure of the chocolate hind, Cephalopholis boenak (Pisces: Serranidae: Epinephelinae), at ping chau island, northeastern Hong Kong waters. Environ. Biol. Fish. 74, 9–18. doi: 10.1007/s10641-005-2258-9
Mackie M. (1993). Reproductive biology and social structure of the blue-spotted rock cod, cephalopholis cyanostigma (Serranidae), and the effects of fishing (Australia: James Cook University).
Mahé K., Rabhi K., Bellamy E., Elleboode R., Aumond Y., Huet J., et al. (2016). Growth of the oblique-banded grouper (Epinephelus radiatus) on the coasts of reunion island (SW Indian ocean). Cybium 40 (1), 61–65. doi: 10.26028/cybium/2016-401-006
Mapleston A., Currey L. M., Williams A. J., Pears R., Simpfendorfer C. A., Penny A. L., et al. (2009). Comparative biology of key inter-reefal serranid species on the great barrier reef (Cairns: Reef and Rainforest Research Centre Limited). Project Milestone Report to the Marine and Tropical Sciences Research Facility.
Marty L., Rochet M.-J., Ernande B. (2014). Temporal trends in age and size at maturation of four north Sea gadid species: cod, haddock, whiting and Norway pout. Mar. Ecol. Prog. Ser. 497, 179–197. doi: 10.3354/meps10580
Molloy P. P., Goodwin N. B., Côté I. M., Reynolds J. D., Gage M. J. G. (2007). Sperm competition and sex change: a comparative analysis across fishes. Evolution 61 (3), 640–652. doi: 10.1111/j.1558-5646.2007.00050.x
Morgans J. F. C. (1982). Serranid fishes of Tanzania and Kenya. Ichthyol. Bull. J.L.B. Smith Inst. Ichthyol. 46, 1–44.
Munro J. L., Williams D. (1985). Assessment and management of coral reef fisheries: biological, environmental and socio-economic aspects. Proc. 5th Int. Coral Reef Congress 4, 545–578.
Nash K. L., Bijoux J., Robinson J., Wilson S. K., Graham N. A. J. (2016). Harnessing fishery-independent indicators to aid management of data-poor fisheries: weighing habitat and fishing effects. Ecosphere 7 (7), e01362. doi: 10.1002/ecs2.1362
Nemeth R. S., Blondeau J., Herzlieb S., Kadison E. (2007). Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ. Biol. Fish 78, 365–381. doi: 10.1007/s10641-006-9161-x
Newman S. J., Williams A. J., Wakefield C. B., Nicol S. J., Taylor B. M., O’Malley J. M. (2016). Review of the life-history characteristics, ecology and fisheries for deep-water tropical demersal fish in the indo-pacific region. Rev. Fish Biol. Fish. 26, 537–562. doi: 10.1007/s11160-016-9442-1
Ohta I., Akita Y., Uehara M., Ebisawa A. (2017). Age-based demography and reproductive biology of three Epinephelus groupers, e. polyphekadion, e. tauvina, and E. howlandi (Serranidae), inhabiting coral reefs in Okinawa. Environ. Biol. Fish. 100, 1451–1467. doi: 10.1007/s10641-017-0655-5
Panfili J., de Pontual H., Troadec J. P., Wright P. J. (2002). Manual of fish sclerochronology. Ifremer-IRD Brest 1, 464. doi: 10.1111/j.0022-1112.2005.0751d.x
Pauly D. (1979). Gill size and temperature as governing factors in fish growth: a generalization of von bertalanffy’s growth formula (Kiel: University of Kiel and Institut für Meereskunde).
Pauly D., Munro J. L. (1984). Once more on the comparison of growth in fish and invertebrates. int. cent. living aquat. resour. manage. Fishbyte 2, 1–21.
Pothin K., Letourneur Y., Lecomte-Finiger R. (2004). Age, growth and mortality of the tropical grouper epinephelus merra (Pisces, serranidae) on reunion island, SW Indian ocean. Vie Milieu. 54, 193–202.
Rachmawati P. F., Puspasari R. (2021). Population parameters of several groupers (Famili serranidae) in labuan bajo waters, East nusa tenggara IOP Conf. Ser.: Earth Environ. Sci. 919, 012001. doi: 10.1088/1755-1315/919/1/012001
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
R Core Team (2021) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available at: https://www.R-project.org/.
Ricker W. E. (1975). Computation and interpretation of the biological statistics of fish populations. Bull. Fish. Res. B. Can. 191, 1–382.
Robinson J., Samoilys M., Kimani P. (2008). “Reef fish sawning aggregations in the western Indian ocean: current knowledge and implications for management,” in CORDIO status report 2007. Eds. Obura D. O., Tamelander J., Linden O. (Indian Ocean, Mombasa: Coastal Oceans Research and Development), 263–276.
Roos D., Aumond Y., Huet J., Bruchon F. (2015). Projet ANCRE-DMX2: Indicateurs biologiques et écologiques pour une gestion durable des stocks de poissons DéMersauX profonds (100–700 m) d’intérêt halieutique à la réunion. Brest, France: IFREMER doi: 10.13155/45812
Roos D., Tessier E., Guyomard D. (1998). Evolution de l'activité halieutique à la réunion de 1990 à 1996 (Le Port (France: Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER). DRV/ RH/RST/98–14.
Sadovy de Mitcheson Y. S., Craig M. T., Bertoncini A. A., Carpenter K. E., Cheung W. W. L., Choat J. H., et al. (2013). Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 14, 119–136. doi: 10.1111/j.1467-2979.2011.00455.x
Sadovy Y., Shapiro D. Y. (1987). Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1, 136–156. doi: 10.2307/1446046
Sakamo Y., Ishiguro M., Kitagawa G. (1986). Akaike information criterion statistics (Netherlands: Springer).
Saleh B., Abozeid M., Ahmed A., Alwany M. A., El-Sherbiny M. M. (2019). Age and growth of epinephelus fasciatus from northern red Sea. Catrina 18 (1) 105–115. doi: 10.21608/cat.2019.28620
Shapiro D. Y. (1987). “Reproduction in groupers,” in Tropical snappers and groupers: Biology and fisheries management. Eds. Polovina J. J., Ralston S. (Boulder: West-view Press), 295–327.
Shapiro D. Y., Sadovy Y., McGehee M. A. (1993). Periodicity of sex change and reproduction in the red hind, epinephelus guttatus, a protogynous grouper. Bullet. Mar. Sci. 53, 1151–1162.
Shpigel M., Fishelson L. (1989). Habitat partitioning between species of the genus cephalopholis (Pisces, serranidae) across the fringing reef of the gulf of aqaba (Red Sea). Mar. Ecol. Prog. Ser. 58, 17–22. doi: 10.3354/meps058017
Sluka R. D. (2001). Grouper and Napoleon wrasse ecology in laamu atoll, republic of Maldives: Part 1. habitat, behavior, and movement patterns. Atoll Res. Bullet. 492, 1–26.
Sluka R., Sullivan K. M. (1996). The influence of habitat on the size distribution of groupers in the upper Florida keys. Environ. Biol. Fish 47, 177–189. doi: 10.1007/BF00005040
Sparre P., Ursin E., Venema S. C. (1987). Introduction to tropical fish stock assessment. part 1: manual (Roma, Italy: FAO Fisheries Technical Paper).
Swainson W. (1839). On the natural history and classification of fishes, amphibians, and reptiles, or monocardian animals. (London: Spottiswoode and Co.) 2, 448.
Takemura A., Rahman M. S., Park Y. J. (2010). External and internal controls of lunar-related reproductive rhythms in fi shes. J. Fish Biol. 76, 7–26. doi: 10.1111/j.1095-8649.2009.02481.x
Thresher R. E. (1984). Coral reef fi shes: reproduction (Hong Kong/Neptune City: T.F.H Publications).
Verhulst P. F. (1838). Notice sur la loi que la population poursuit dans son accroissement. Corresp. Math. Phys. 10, 113–121. doi: 10.1371/journal.pbio.1001827
Vitale F., Worsøe Clausen L., Ní Chonchúir G. (2019). Handbook of fish age estimation protocols and validation methods. ICES Coop. Res. Rep. 346, 1–180. doi: 10.17895/ices.pub.5221
Von Bertalanffy L. (1938). A quantitative theory of organic growth (Inquiries on growth laws II). Hum. Biol. 10, 181–213.
Wahbeh M. I. (2005). Some aspects of reproduction and growth of the grouper, cephalopholis miniata (Forsskål), the blacktip grouper, epinephelus fasciatus (Forsskål), and the lunartail grouper, variola louti (Forsskål) from the north-eastern coast of the gulf of aqaba (Red Sea), Jordan. Dirasat Pure Sci. 32 (2), 171–182.
Weatherley A. H. (1990). Approaches to understanding fish growth. Trans. Am. Fish. Soc 119, 662–672. doi: 10.1577/1548-8659(1990)119<0662:ATUFG>2.3.CO;2
Williams D. (1991). “Patterns and processes in the distribution of coral reef fishes,” in Ecology of fishes on coral reefs. Ed. Mora C. (Cambridge: Cambridge University Press), 104–115. doi: 10.1017/CBO9781316105412.013
Keywords: La Réunion Island, Variola, growth, reproduction, Indian Ocean, Epinephelus, Cephalopholis, sexual maturity
Citation: Mahé K, Gentil C, Brisset B, Evano H, Lepetit C, Boymond-Morales R, Telliez S, Dussuel A, Rungassamy T, Elleboode R, MacKenzie K and Roos D (2022) Biology of exploited groupers (Epinephelidae family) around La Réunion Island (Indian Ocean). Front. Mar. Sci. 9:935285. doi: 10.3389/fmars.2022.935285
Received: 03 May 2022; Accepted: 03 October 2022;
Published: 21 October 2022.
Edited by:
Pierluigi Carbonara, COISPA Tecnologia & Ricerca, ItalyReviewed by:
Andrea Bellodi, University of Cagliari, ItalyJorge Paramo, University of Magdalena, Colombia
Copyright © 2022 Mahé, Gentil, Brisset, Evano, Lepetit, Boymond-Morales, Telliez, Dussuel, Rungassamy, Elleboode, MacKenzie and Roos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kélig Mahé, kelig.mahe@ifremer.fr
 Kélig Mahé
Kélig Mahé Claire Gentil1,2
Claire Gentil1,2  Kirsteen MacKenzie
Kirsteen MacKenzie David Roos
David Roos