Megacraniinae, Hennemann, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4896.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:0BAD0251-42BC-4C88-BDDC-9622FD1F9F13 |
|
DOI |
https://doi.org/10.5281/zenodo.4384019 |
|
persistent identifier |
https://treatment.plazi.org/id/2570264F-B334-FFB1-98BC-F904FC8BFE64 |
|
treatment provided by |
Plazi |
|
scientific name |
Megacraniinae |
| status |
subfam. nov. |
Megacraniinae View in CoL subfam. nov.
Type-genus: Megacrania Kaup, 1871: 38 View in CoL .
Platycraniae Brunner v. Wattenwyl, 1893: 97 (in part - not the type-genus Platycrana Gray, 1835 View in CoL ).
Redtenbacher, 1908: 368 (in part). [ Phibalosomini (II. Sectio Platycraniae]
Platycraniinae Günther, 1953: 557 (in part). [Incorrect spelling based on misspelled Platycrania, Westwood, 1859 View in CoL ] Platycraninae Bradley & Galil, 1977: 190 View in CoL (in part).
Otte & Brock, 2005: 33 (in part).
Hennemann & Conle, 2008: 24, 26, 57 (in part).
Acrophyllinae Kirby, 1904: 379 (in part).
Cladoxerinae Karny, 1923: 237 (in part).
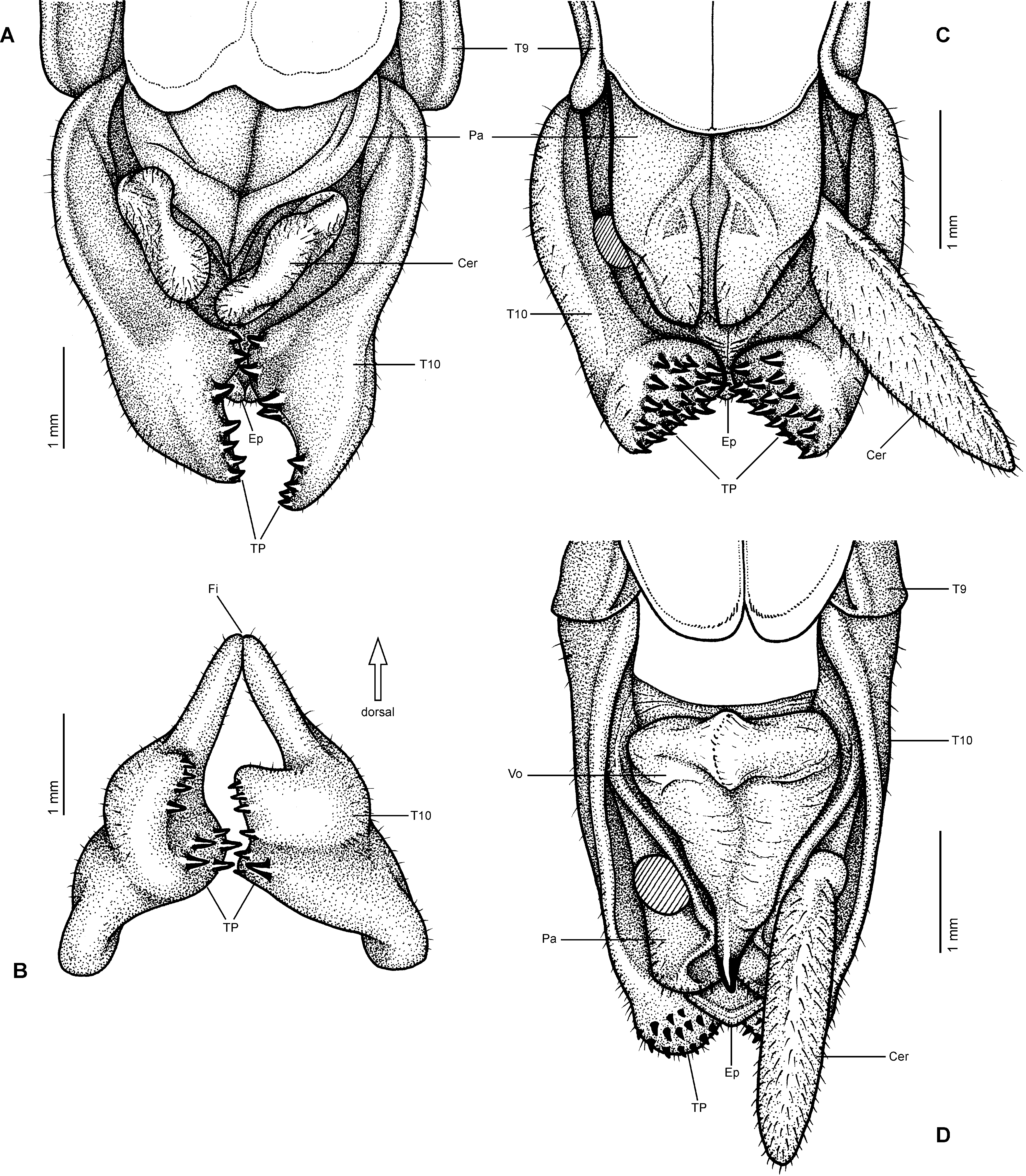
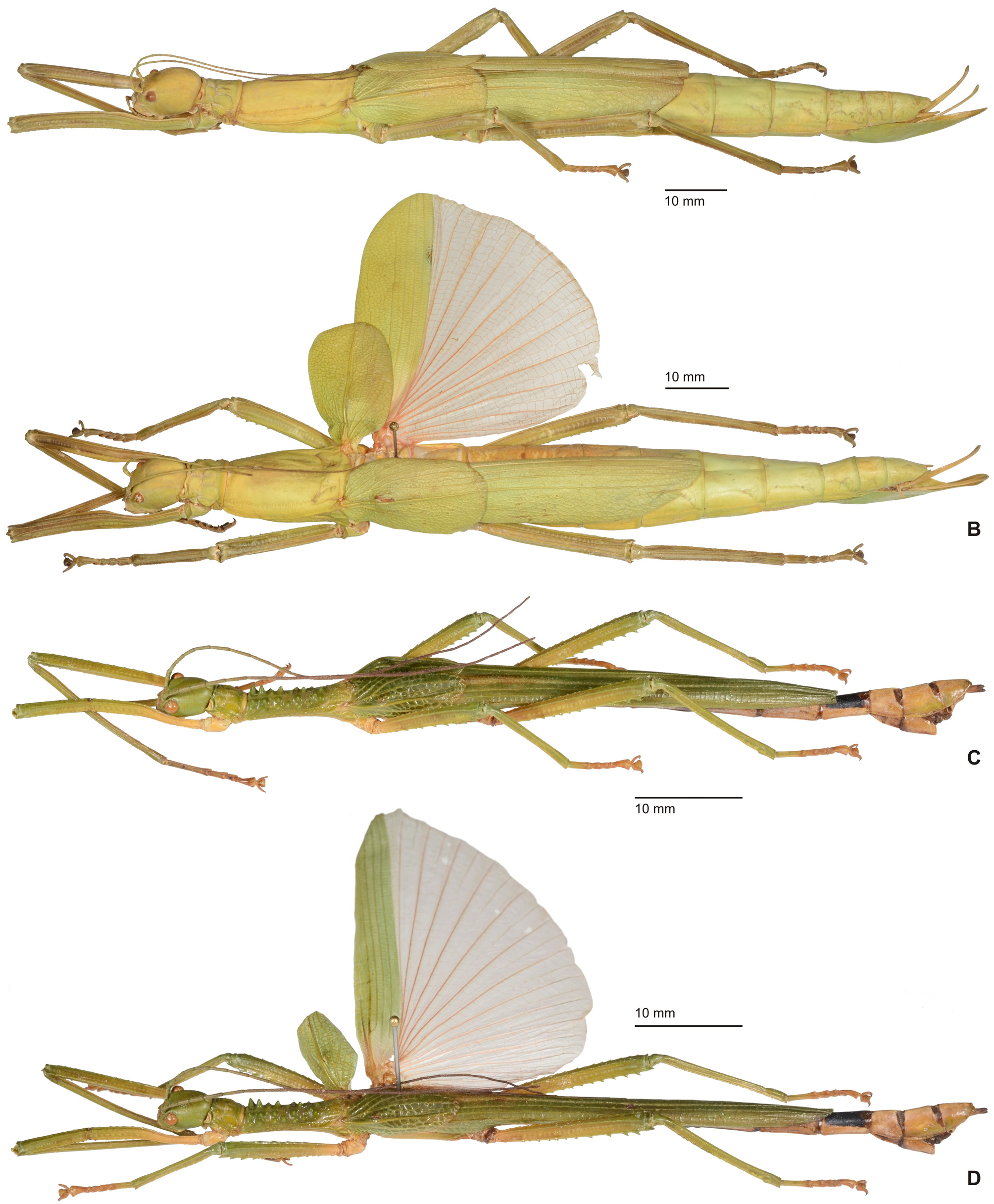
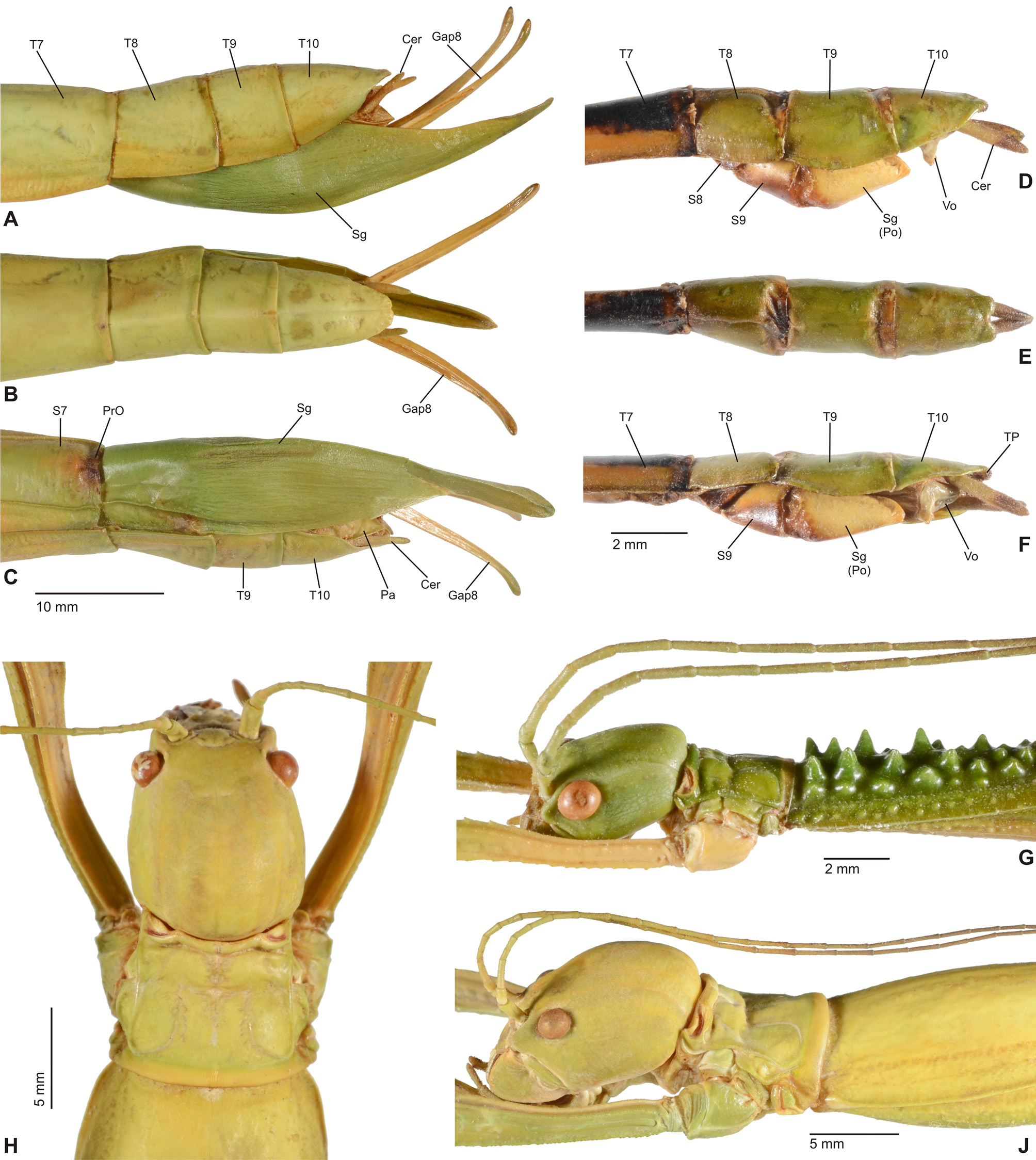
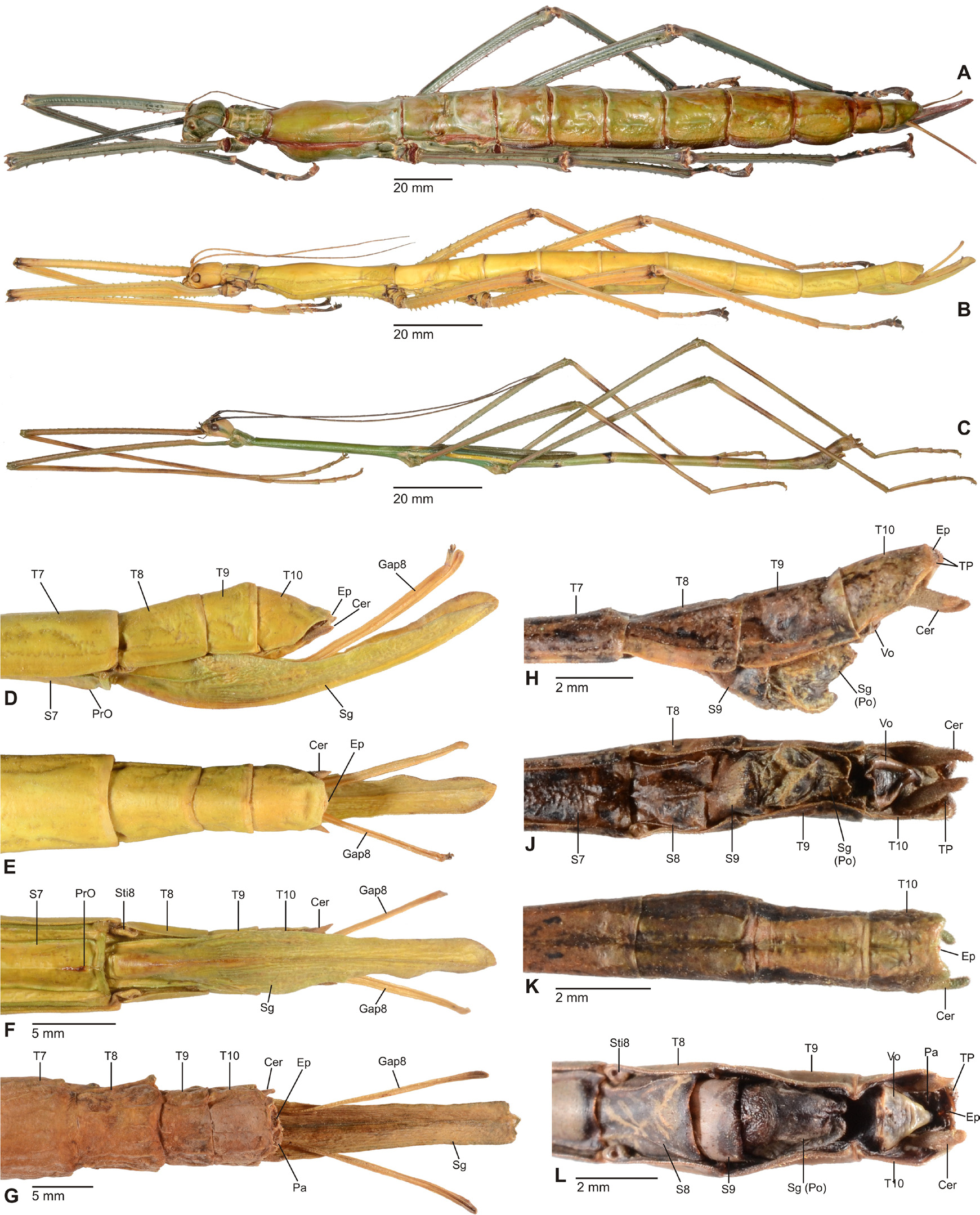
Diagnosis (♂, ♀): Small to medium-sized (body length 41.5-132.0 mm) often very colourful Phasmatidae , form ranging from slender to fairly robust; body sub-cylindrical. ♂♂ apterous ( Fig. 3D View FIGURE 3 ), brachypterous or with well developed alae ( Figs. 1 View FIGURE 1 B–C, 2A, 3A–C), ♀♀ apterous, brachypterous ( Figs. 2B, D View FIGURE 2 ) or with moderately developed alae ( Figs. 1A View FIGURE 1 , 2C View FIGURE 2 ). Sexual dimorphism moderate. Head conspicuously enlarged, notably broader and longer than prothorax and flattened dorsoventrally, vertex smooth and with a distinct longitudinal postocular furrow laterally ( LCF); genae strongly enlarged ( Fig 4 View FIGURE 4 A-J). Eyes more or less decidedly displaced towards the dorsal surface of the head ( Fig. 4 View FIGURE 4 A-J). No ocelli. Gula large and covering> ½ of cervical region ( Fig. 5 View FIGURE 5 A-B). Lacinia usually with three terminal teeth. Antennae moderately slim to robust and often perlamorph, distinctly segmented and at best equal in length to profemora (much shorter than profemora in ♀♀); consisting of less than 30 segments. Two basal antennomeres flattened dorsoventrally, the scapus moderately broadened. Pronotum with well developed, often very prominent defensive glands at anterolateral corners; these lateral directed ( Figs. 4 View FIGURE 4 A-J). Mesothorax> 2x longer than prothorax; parallel-sided and more or less decidedly compressed dorsoventrally; mesonotum usually more or less flattened, mostly smooth to sparsely granulose ( Fig. 4G View FIGURE 4 ) but may be tuberculose ( Fig. 4A View FIGURE 4 ) to spinulose in certain taxa ( Fig. 4C View FIGURE 4 ), with a fine medio-longitudinal carina. Tegmina flat and spatulate; in winged taxa notably shorter than alae. Anal fan of alae transparent, plain grey, pink or orange; only in one known case with a darker outer margin. Median segment longer than metanotum. Abdomen excluding median segment considerably longer than head and complete thorax combined. Abdominal segments II-VI longer than wide. No praeopercular organ on abdominal sternum VII of ♀♀ (Fig.. 8E). Terminalia of ♂♂ ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 ): Anal segment tectiform and split longitudinally to form two movable hemi-tergites that are dorsally connected by a narrow fissure (FI, Figs. 7B, E View FIGURE 7 , G-J); interior surface of hemi-tergites with various specializations, either with paired medio-ventral directed thorn-pads ( Fig. 6C View FIGURE 6 ), or with often asymmetrical rows of teeth or dentate ridges that are directed medially against each other ( Figs. 6A, 6B View FIGURE 6 , 7B, 7E View FIGURE 7 ). No vomer ( Figs. 6A, C View FIGURE 6 ). Paraprocts variable. Poculum (= subgenital plate) small, rather flat to moderately convex and scoop-shaped ( Figs. 7A, C, D, F View FIGURE 7 ). Terminalia of ♀♀ ( Fig. 8 View FIGURE 8 ): Subgenital plate at best slightly projecting beyond but mostly not reaching to apex of abdomen, obtusely convex to moderately keeled longitudinally; often with a knob-, wart-, or transversely ridge-like basal swelling (here referred to as “opercular organ”, Figs. 8 View FIGURE 8 A-E). Gonapophysis VIII more or less equal in length to gonapophysis IX, gonoplac variable in size, sometimes notably enlarged ( Fig. 8A View FIGURE 8 ) and occasionally fused with gonapophysis IX; all hidden within subgenital plate and not reaching to apex of abdomen. Gonangulum present but small. Cerci in both sexes compressed laterally, more or less enlarged and elongated, foliaceous or lanceolate in shape ( Figs. 6-8 View FIGURE 6 View FIGURE 7 View FIGURE 8 ). All femora and tibiae trapezoidal in crosssection; tibiae usually less decidedly carinate. Tibiae without an area apicalis. Profemora somewhat constricted and strongly curved in basal portion ( Figs. 4 View FIGURE 4 A-E); dorsal carinae very slightly nearing, the posterodorsal carina at best very slightly raised sub-basally and occasionally minutely denticulate. Medioventral carina of all femora midways on ventral surface and often set with a variable number of small spines or teeth. Extrimities otherwise unarmed. Basitarsi slender, not lobed dorsally .
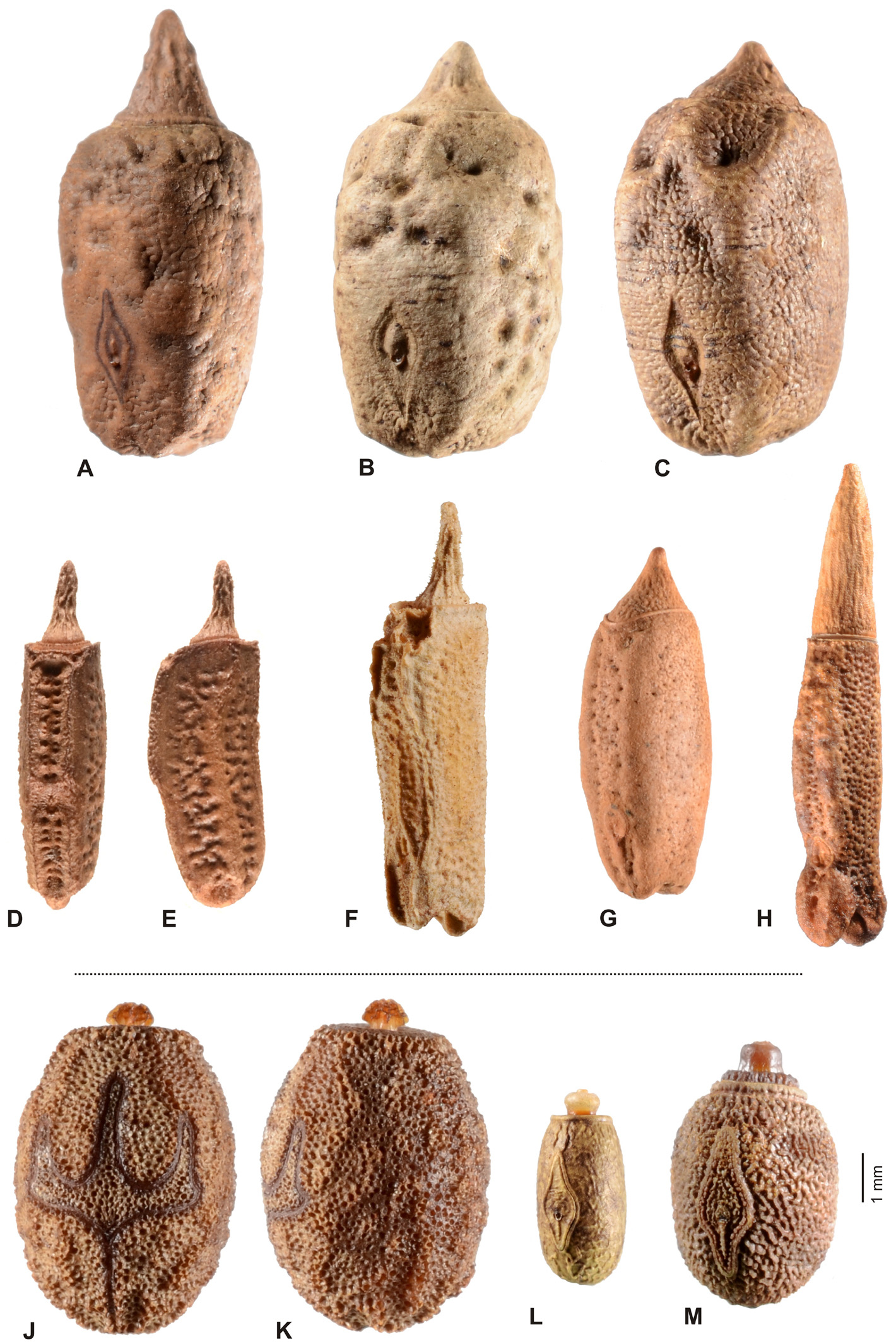
Eggs ( Figs. 12 View FIGURE 12 A–H): Medium-sized to very large, more or less alveolar and /or polygonal; capsule distinctly longer and higher than wide. Dorsal surface often strongly convex. Polar area mostly more or less distinctly indented. Capsule surface prominently sculptured, being granulose, tuberculose, rugulose, coriaceous or punctured; usually with at least weak irregular longitudinal ridges and/or keels. Micropylar plate small, less than ½ the length of capsule and notably displaced towards the posterior end of capsule; shape more or less rhomboidal. Micropylar cup placed roughly in centre of plate. Internal micropylar plate closed. Operculum strongly raised and conical or peg-like.
Comments: The subfamily Platycraninae was originally established by Brunner v. Wattenwyl (1893: 97) as Platycraniae to include the genera Platycrana Gray, 1835 , Graeffea Brunner v. Wattenwyl, 1868 and Arrhidaeus Stål, 1877 (= Ophicrania Kaup, 1871 ). Platycrana is the genus-eponymum and hence the type-genus. Subsequent authors (e.g. Günther, 1953; Bradley & Galil, 1977) traditionally characterized the subfamily mainly by the morphological character combination “Cheeks broader than eye” and “Wings, but the tegmina in particular, shortened or lacking”.
However, Platycrana Gray, 1835 ( Figs. 9-10 View FIGURE 9 View FIGURE 10 ) the type-genus of Platycraninae , differs fundamentally from all other genera currently attributed to the subfamily in numerous morphological characteristics and does not even exhibit the aforementioned main diagnostic characteristic of the subfamily, the distinctly enlarged genae (or “cheeks”). Hence, the subfamily in its present recognition is obviously polyphyletic. This has already been pointed out and illustrated by Bradler (2001: 181, fig. 2) and Bradler (2009: 63, fig. 33), who studied the external genitalia of ♂♂, as well as by Hennemann & Conle (2008: 24, 26), who in their distinguishing key to the subfamilies and tribes of Phasmatidae s. str. (= Lanceocercata Bradler, 2001) referred to “ Platycraninae ” as comprising all genera attributed to the subfamily at that time except for the type-genus Platycrana itself. In addition to stating that Platycrana was not closely related to the remaining genera of Platycraninae in its traditional recognition, Hennemann & Conle (2008: 57) suggested close relation between Platycrana and the tribe Stephanacridini Günther, 1953 ( Fig. 11 View FIGURE 11 ). Bradler (2001: 181, fig. 2; 2009: 63, fig. 33) provided figures of the genitalia of ♂♂ of Platycrana and two members of Megacraniinae subfam. nov., that clearly illustrate the fundamental differences in genital morphology. In ♂♂ of Platycrana the anal segment is flat and entire with the posterior margin just gently indented and the vomer is well developed as a roughly triangular, strongly sclerotised plate that bears a terminal hook ( Fig. 6D View FIGURE 6 ). The cerci are small, cylindrical and do not show any specializations. All other genera formerly attributed to Platycraninae , and here comprised in Megacraniinae subfam. nov., have the anal segment more or less tectiform and split longitudinally into two movable hemi-tergites that bear variable dentate specializations on their interior surface to form a clasping apparatus analogous to that of other Phasmatidae s. str. (= Lanceocercata, Figs. 6-7 View FIGURE 6 View FIGURE 7 ). Also the genital morphology of ♀♀ of these two clades shows fundamental differences. In Platycrana the subgenital plate is much elongated and projects considerably beyond the apex of the abdomen, there is a praeopercular organ on abdominal sternum VII but no analogous structure at the base of the subgenital plate as in Megacraniinae subfam. nov., the cerci are small and cylindrical, and the gonapophysis VIII are extremely elongated, filiform and project greatly beyond the apex of the abdomen ( Figs. 10 View FIGURE 10 A-C). In all other genera formerly attributed to Platycraninae , and here comprised in Megacraniinae subfam. nov., the subgenital plate hardly projects over the apex of the abdomen and bears an opercular organ (OpO) that is formed by a knob-, wart-, or transversely ridge-like swelling at the base ( Figs. 8 View FIGURE 8 A–E) and serves as an anchorage for the ♂♂ clasping apparatus during copulation, the cerci are laterally compressed, more or less elongated and foliaceous or lanceolate ( Figs. 6-8 View FIGURE 6 View FIGURE 7 View FIGURE 8 ), and the gonapophysis VIII are not notably elongated and fully hidden within the subgenital plate. Furthermore, the main key feature of Platycraninae sensu Günther, 1953 , the strongly enlarged genae (“cheeks”) only matches for the genera here comprised in Megacraniinae subfam. nov.. These have the genae strongly enlarged to accommodate the massive mandibular muscles necessary for feeding on the hard leaves of various palms ( Arecaceae ) e.g. coconut ( Cocos nucifera ) or screw palms ( Pandanus spp., Pandanaceae ). The head in these taxa is remarkably large, flattened dorsoventrally and the eyes are more or less distinctly displaced towards the dorsal surface of the head capsule ( Fig. 4 View FIGURE 4 ). Moreover, the head capsule bears a distinct longitudinal postocular furrow here referred to as the lateral cervical furrow (LCF, Fig. 4 View FIGURE 4 ). In contrast, Platycrana has a fairly usual phasmatodean head, that is ovoid and somewhat widened towards the posterior with the vertex roundly convex and the eyes placed on the lateral surfaces of the head capsule ( Figs. 10 View FIGURE 10 H-J). Finally, also the eggs of Platycrana are unlike those of all other genera of Platycraninae sensu Günther, 1953 (here comprised in Megacraniinae subfam. nov.), being ovoid with a flat operculum that bears a small knob-like capitulum in the centre and having a large micropylar plate which exhibits two conspicuous lateral hook-like expansions ( Figs. 12 View FIGURE 12 J-K). All genera in Megacraniinae subfam. nov. have more or less alveolar and polygonal eggs that bear a large, conical to peg-like operculum and have a fairly small mostly rhomboidal micropylar plate ( Figs. 12 View FIGURE 12 A-H).
As already suggested by the distinctive genital morphology of ♂♂ in particular, not only phylogenetic approaches based on morphological characters (e.g. Bradler, 2009) but also molecular-based phylogenetic studies (Buckley et al., 2009; Bradler et al., 2015) have frequently revealed Megacraniinae subfam. nov. (in all mentioned studies referred to as “ Platycraninae ”) as a subordinate clade of Phasmatidae s. str. (= Lanceocercata). Thus Platycrana , and the subfamily Platycraninae sensu novo consequently, are here removed from Phasmatidae s. str. (= Lanceocercata), and based on morphological characters shown to be closely related to Stephanacridini (+ see discussion of Platycraninae below). Molecular studies (Buckley et al., 2009) have recovered Stephanacridini as the sister group of Phasmatidae s. str. (= Lanceocercata) and in removing Platycraninae from this clade, Stephanacridini becomes a subordinate taxon of Platycraninae sensu nov. . Consequently and in accordance to the results of Buckley et al. (2010), Platycraninae instead of Stephanacridini can now be considered as the sister group of Phasmatidae s. str. (= Lanceocercata; Fig. 13 View FIGURE 13 ). A detailed discussion of Platycraninae sensu novo and the arrangement of tribes and genera within the subfamily is presented below.
Given that Platycrana , the type-genus of Platycraninae , is not closely related to any of the other genera traditionally attributed to that subfamily a new subfamiliar name is necessary to accommodate these genera. In order to emphasize the main key feature of this subfamily, i.e. the remarkably enlarged head and genae, Megacraniinae subfam. nov. is here introduced with Megacrania Kaup, 1871 as the type-genus. This new subfamiliar name, a combination of the Greek μέγας or mega (= large) and κραΝΊΟΝ or cranium (= skull), is chosen because it very well describes the main key characteristic that distinguishes this subfamily from all other related taxa. The vernacular name “Palm stick insects” is here preferred to “Coconut Stick Insects”, as used by Bradler et al. (2015), because members of this subfamily are not exclusively feeding on coconut palms but are known to feed on a variety of different Arecaceae (e.g. Caryota spp., Cycas spp., Cocos nucifera , Trachycarpus spp., Metroxylon spp.) and screw palms of the family Pandanaceae .
It appears noteworthy, that among Phasmatidae s. str. (= Lanceocercata) members of Megacraniinae subfam. nov. show two striking morphological features that otherwise are exclusive to genera of the New Zealand clade Acanthoxylini Günther, 1953 . The opercular organ (OpO) is a conspicuous swelling at the base of the ♀♀ subgenital plate that serves as an anchorage for the ♂♂ clasping apparatus during copulation. It varies considerably in size, shape and sculpturing among the species and genera of Megacraniinae subfam. nov. ranging in shape from knob-, over wart-like to forming an obtuse transverse ridge. While this structure is very prominent in e.g. Megacrania Kaup, 1871 ( Figs. 8 View FIGURE 8 C-E), Erastus Redtenbacher, 1908 ( Fig. 8A View FIGURE 8 ) and Graeffea Brunner v. Wattenwyl, 1868 ( Fig. 8B View FIGURE 8 ), it is just weakly developed to almost obsolete in Ophicrania Kaup, 1871 . Being analogous to the structure in Acanthoxylini it is seen to be similarly variable in this latter clade (see Buckley, Myers & Bradler, 2014: 460, fig. 4). The presence of an opercular organ could be a synapomorphy of Megacraniinae subfam. nov. + Acanthoxylini , but further evaluation is needed to confirm this assumption. Another possible synapomorphy of these two clades is exhibited by the eggs. In contrast to all other members of Phasmatidae s. str. (= Lanceocercata), which show a true capitulum, the eggs of Megacraniinae subfam. nov. and Acanthoxylini have a more or less prominently raised and conical or peg-like operculum ( Figs. 12 View FIGURE 12 A-H), which may be as high as more than half the length of the egg capsule in some extreme cases (e.g. some species of Ophicrania ). Moreover, eggs of both clades are more or less polygonal with the dorsal surface bulgy longitudinally and the polar-area more or less prominently indented. It shall bmen- tioned that there have been contrary definitions of the opercular structures of genera of Megacraniinae subfam. nov. in the past. According to Clark Sellick (1988: 278) the eggs of Megacrania and Ophicrania have “raised spongy opercula” but according to Clark Sellick (1997: 118, figs. 71-72) capitula are present in the two genera. Since a true capitulum is defined as a structure that is easily detached from the operculum without damage to the latter and with its loss not having negative influence on the hatching rate ( Clark, 1976; Clark Sellick, 1988), there is no capitulum in Megacraniinae subfam. nov..
A detailed differentiation and comparison between Platycrana Gray, 1853 and Megacraniinae subfam. nov. is presented in table 1 below.
Genera excluded from Megacraniinae : The Australian Echetlus Stål, 1875 (Type-species: Bacillus peristhenes Westwood, 1859: 13 , pl. 7: 1) was included in former Platycraninae by Günther (1953: 557) and has since been retained in the subfamily by all subsequent authors. Brock & Hasenpusch (2007: 74) and Hennemann & Conle (2008: 28) doubted Echetlus to be a member of Platycraninae sensu Günther and stated it was likely to belong in Phasmatinae . Indeed, Echetlus is obviously not at all closely related to either Platycrana , and the subfamily Platycraninae sensu nov. respectively, nor to any of the genera of Megacraniinae subfam nov. . Consequently, since the genus does not have any of the characteristics and can hence not be incorporated in either Platycraninae sensu nov. or Megacraniinae subfam. nov., it is here provisionally transferred to Phasmatinaea: Acanthomimini . Morphological features such as the slender and stick-like habitus, the characteristic profemora which have the anterodorsal carina strongly raised and serrate and are triangular in cross-section, the elongate and cylindrical head as well as short and stocky antennae that are considerably shorter than the profemora and also the geographic distribution in Western Australia suggest close relation to genera currently in Acanthomimini . Hennemann & Conle (2008: 28) also pointed out that the the Brazilian species attributed to Echetlus by Zompro (2004) is not congeneric and is a member of the New World Diapheromeridae : Diapheromerinae .
Redtenbacherus Özdikmen & Darilmaz, 2008 was introduced as a replacement name for the preoccupied Ernodes Redtenbacher, 1908 (Type-species: Ernodes sumatranus Redtenbacher, 1908: 374 ). In discussing Redtenbacherus and transferring all species except for the type-species to Lopaphus Westwood, 1859 (subfamily Necrosciinae ), Seow-Choen (2018: 450) commented “ This species appears to be a Lopaphus species […]. The antennae appear damaged, and therefore artificially shortened. It is likely to be proven to belong to that genus when more information is available. ”. Indeed, R. sumatranus strongly resembles certain species of Lopaphus and the species has none of the characteristic features of either Platycraninae sensu nov. or Megacraniinae subfam. nov.. If taking broken antennae into account in the unique holotype, the slender and cylindrical body and stick-like habitus, entirely unarmed extremities, triangular cross-section of the profemora, which have the anterodorsal carina strongly raised, morphology of the head and genitalia clearly place this genus in the subfamily Necrosciinae . Consequently, and since it cannot be retained in either Platycraninae nor Megacraniinae , Redtenbacherus is here transferred to Necrosciinae .
The genus Elicius Günther, 1935 (Type-species: Elicius microbasileus Günther, 1935: 16 ) was described from a unique male from Mount Latimojong, South Sulawesi. Already Günther (1935: 16) was in doubt about the systematic position and relationships of this tiny species and placed it in Platycraninae with reservation. The author commented that the position of the genus within Platycaninae was just as incoherent as if it had been placed in the subfamily Necrosciinae . However, detailed examination of the holotype in the Museum für Naturkunde Berlin, Germany (MNHU) proves that Elicius is not a member of Megacraniinae subfam. nov. (= Platycraninae sensu Günther ). This is seen by morphological features such as the ovoid head that has the large eyes positioned laterally, the genae not notably enlarged and lacks a lateral cervical furrow, the small gula, tectiform but entire anal segment, presence of a well-developed vomer, cylindrical cerci, and long antennae that are almost as long as the front legs. These features place Elicius in the subfamily Necrosciinae to which it is here transferred. The enlarged head as well as the smooth and shiny body surface resemble certain Wallacean members of Paranecroscia Redtenbacher, 1908 and the genital morphology is similar to e.g. Moritasgus Günther , 19035, both members of the Necrosciinae .
Natural History: Many papers have dealt with the biology (e.g. Franzman, 1974; Bedford, 1978; Preston-Mafham, 1990; Rapp, 1995; Cermak & Hasenpusch, 2000), ecology, host plants and pest status (e.g. Baker, 2015; Deesh et al., 2020), population dynamics and dispersal ( Kobayashi et al., 2014; Kobayahshi et al., 2016) and chemical defensives (e.g. Smith et al., 1979; Chow & Lin, 1986; Ho & Chow, 1993; Jones & Bulbert, 2020) of members of this particular subfamily. Natural host plants comprise various palms of the families Arecaceae and Pandanaceae . For example, the so-called “Peppermint Stick Insect” Megacrania batesii Kirby, 1896 is reported to feed on Pandanus tectorius , P. monticola and P. soloms-laubachii in coastal parts of North Queensland, Australia ( Cermak & Hasenpusch, 2000). The closely related Megacrania tsudai Shiraki, 1933 also feeds on Pandanus tectorius in similar habitats in Taiwan ( Chow & Lin, 1986). Several species, including Graeffea crouanii (Le Guillou, 1841) , G. minor Brunner v. Wattenwyl, 1868, G. lifouensis Sharp, 1898 , G. leveri (Günther, 1937) and Acanthograeffea denticulata ( Redtenbacher, 1908) feed predominantly on coconut ( Cocos nucifera , Arecaceae ) in their natural habitats. While Acanthograeffea denticulata has also been recorded on a species of Pandanus in the Marianas ( Muniappan, 2002), Graeffea crouanii has been observed to feed on sago ( Metroxylon sagu , Arecaceae ; Bedford, 1978) and the grass Miscanthus floridulus (Poaceae) in Fiji ( Lever, 1947). In captivity in Europe various palms of the Arecaceae family are frequently accepted as substitutes by species of the genera Graeffea Brunner v. Wattenwyl, 1868 and Ophicrania Kaup, 1871 . These include Dypsis lutescens , Howea forestiana , Trachycarpus fortunei , Phoenix canariensis and Livistona australis but also bamboo (Phyllostachyus aurea, Poaceae ) was readily taken by G. crouanii in captivity (Bruno Kneubühler, pers. comm.). Although Megacrania tsudai causes considerable damage to Pandanus screw palms in Taiwan (e.g. Chow & Lin, 1986) and M. batesii to the same host plants in coastal parts of Northeast Australia ( Cermak & Hasenpusch, 2000), these damages are not of economic importance. The so-called “Coconut Stick Insect” Graeffea crouanii however, is for long known to defoliate coconut palms ( Cocos nucifera ) in many islands of the South Pacific and to be of great economic importance. Since coconut palms are one of the most important crops and the coconut industry is the most important agricultural commodity for many smaller islands throughout the Pacific, it plays important roles in the livelihoods of people in terms of food and nutrition security and economic aspects. Hence, a decrease in infestation of coconut palms by G. crouanii is mandatory for the survival and boost of the coconut industry ( Deesh et al., 2013). The worst damage is mostly seen on old trees that are at least 25 m in height and in times of severe damage, frequently whole plants are defoliated and die ( Swaine, 1969). For example, Paine (1968) reported a severe outbreak on the island of Taveuni, Fiji in 1958-1959 and extending into 1961 with over 500 acres of coconut palms affected of which at least 50% of the older fronds were completely defoliated and almost 400 palms killed. Many species of Megacraniinae subfam. nov. are found in coastal regions, which is mostly due to the distribution and occurrence of their host plants. Kobayashi et al. (2014) suggested a dispersal of eggs through seawater to be a possible distributional mechanism of Megacrania tsudai and tested this hypothesis in laboratory conditions. The tests provided support for this assumption because eggs were shown to have specific characteristics that allow their survival when they float in the sea and that the hatching rates were not negatively affected. The same seawater resistance and dispersal by floating was reported for Graeffea crouanii in the South West Pacific ( Swaine, 1969). Members of the subfamily are also characteristic for having well developed bilateral prothoracic defensive glands, which have their openings at the anterior corners of the pronotum. From these the insects can spray good amounts of a white, milky and often strongly smelling and irritating secretion towards potential predators. The secretion does not effect human skin (Franzmann, 1974) but irritates the eyes on contact ( Cermak & Hasenpusch, 2000). In species of Megacrania the smell resembles peppermint (e.g. Franzmann, 1974) and in Graeffea leveri the smell is described as lemon-like (Bruno Kneubühler, pers. comm). Gas chromatography-mass spectrometry (GC-MS) analyses of the defensive secretion of Megacrania tsudai in Taiwan have shown it’s major component to be actinidine but also to contain boschniakine and two stereoisomeres of 1-acetyl-3-methylcloptentane ( Ho & Chow, 1993).
Distribution: Oriental Region from Japan and Taiwan in the north over Peninsular Malaysia, Borneo and the Philippines towards Wallacea, the Papuan subregion and NE-Australia, and throughout the South West Pacific in Melanesia, southern Micronesia, New Caledonia and as far South East as French Polynesia. One species is endemic to the Seychelles and one possibly extinct genus, Xenomaches Kirby, 1896 , was described from Rodrigues Island, both in the Indian Ocean.
| LCF |
I.N.T.A. |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Megacraniinae
| Hennemann, Frank H. 2020 |
Cladoxerinae
| Karny, H. H. 1923: 237 |