
Cookiecutter sharks (Isistius) are a strong contender for one of the strangest sharks alive today. These small-bodied sharks – usually under half a meter in length – pack a daunting lower dentition consisting of proportionally massive (especially noticeable in I. plutodus), blade-like teeth that facilitates their infamous and bizarre lifestyle as ectoparasitic predators, biting out plugs of flesh from much larger fish – sharks, marlins, tuna, etc. – and marine mammals, namely seals and whales. This feeding method leaves behind circular wounds in the victim’s skin, aptly giving the cookiecutter shark its common name. Regarding its fossil record, the oldest teeth of Isistius are currently from the Cenozoic (Cappetta, 2012; de Figueiredo Petean & de Carvalho, 2018), so evidence is lacking on whether they were around in the Mesozoic to pester the many marine reptiles and sharks alive then. But there is a Late Cretaceous shark, recently described on the basis of highly distinctive teeth, that may have led a similar lifestyle: Hessinodon wardi.

Hessinodon wardi was described from four lower jaw teeth by Cappetta, Morrison, & Adnet (2021), which reported on a diverse assemblage of shark teeth from the upper Campanian Northumberland Formation, a part of the larger Nanaimo Group exposed in Vancouver Island and nearby areas. Two teeth were collected from Collishaw Point and the other two from Manning Point; both localities are located on the northwestern coast of Hornby Island, situated just to the east of Vancouver Island. The genus was named after Ontarian paleontologist William A. Hessin – “in recognition of his extensive work on Canadian trilobites” (Cappetta, Morrison, & Adnet, 2021:1159) – and Beatrice R. Hessin, and the species after paleontologist Peter D. Ward from the University of Washington, “in recognition of all his work as a specialist in Nanaimo Group ammonites/nautiloids and recent nautilus, documenting the biostratigraphy of Hornby Island and especially for his help and encouragement during the collection of shark material on Hornby Island” (Cappetta, Morrison, & Adnet, 2021:1159).

Systematics
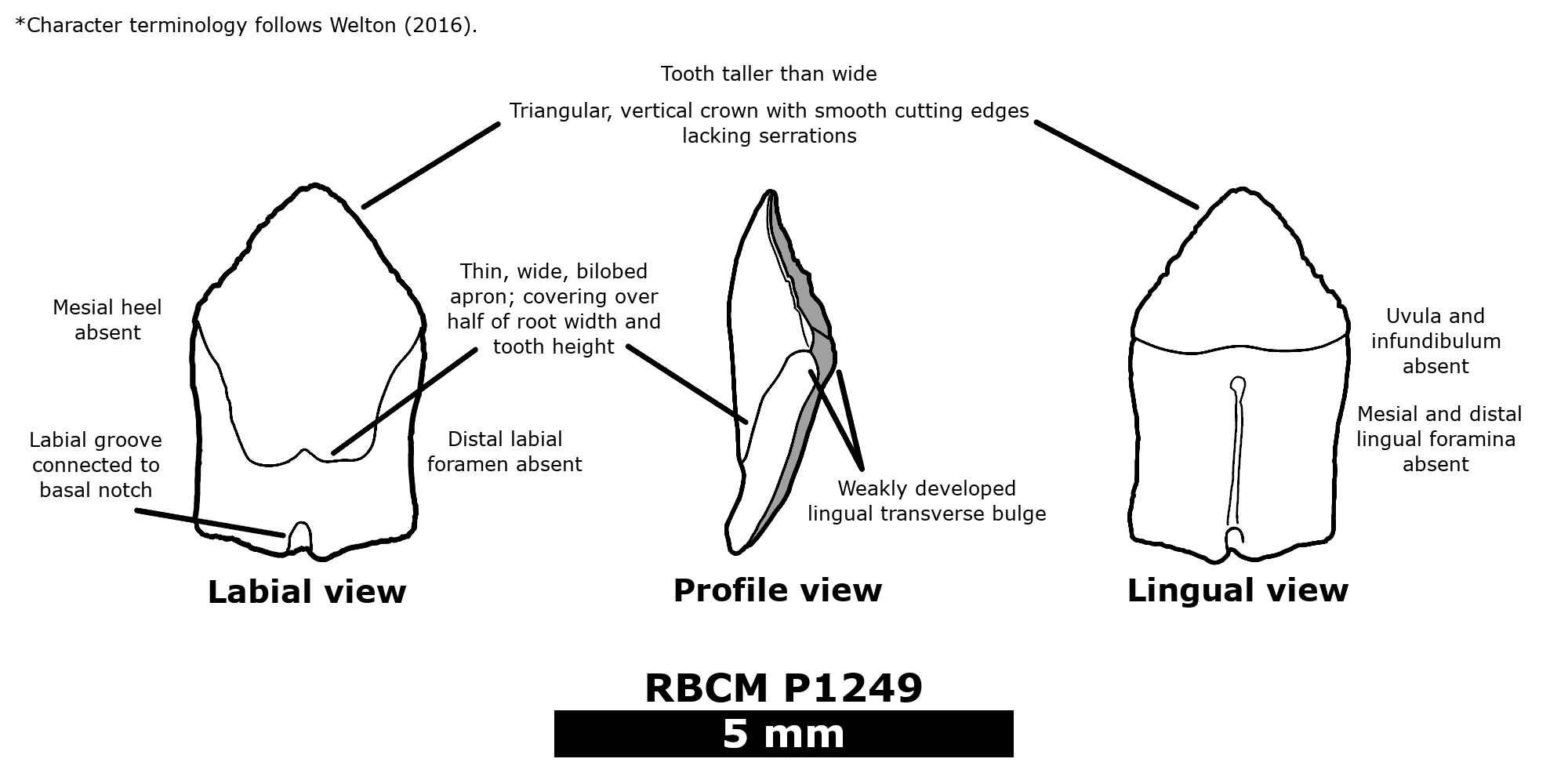
The lower teeth of Hessinodon wardi are very distinctive from many other squaliforms in morphology, and indeed nothing of its likeness had been found from the Mesozoic before. They are symmetrical, with crowns that have smooth-cutting edges and form an isosceles triangle. Although superficially resembling roughshark (Oxynotus) teeth, the lower dentition of Hessinodon is closer to Isistius with their symmetrical shape and triangular crown with smooth edges. It was presumably on this basis that Cappetta, Morrison, and Adnet tentatively suggested Hessinodon belonged to Dalatiidae. They do not explicitly discuss this referral besides how the absence of evidence of strong dignathic heterodonty – the condition where the upper and lower dentitions exhibit great morphological disparity – in Hessinodon makes its referral to Dalatiidae difficult, considering that it is present in all other members. When using the familial diagnosis of Welton (2016) for distinguishing dalatiid dentition from other squaliform families, the combination of numerous characters listed is observable in Hessinodon (labelled below). In conjunction with its similarities to Isistius, considering it as a dalatiid is currently appropriate. It is worth mentioning that Hessinodon lacks a “button-hole” in its lower teeth, characteristic of Isistius and Dalatias (Cappetta, 2012; Welton, 2016), so if the former is closely related to the clade the latter two commonly form (Naylor et al. 2012; Straube et al. 2015; Denton et al. 2018; Pollerspöck & Straube, 2021; Feichtinger et al. 2022), it appears to be basal to them. So far, the referral of Hessinodon to Dalatiidae has not been contested (Feichtinger et al. 2022), but considering its unique dentition, further remains and work are needed to fully assess its affinities.

Appearance and Size
Being only known from teeth, there is no evidence for the appearance of Hessinodon in life. Considering that each extant dalatiid is morphologically unique and specialized, predicting the external anatomy of Hessinodon with any certainty is difficult. However, assuming that it is closely related to Isistius and Dalatias by virtue of their similar dental morphologies, reconstructing the general form of Hessinodon becomes somewhat easier by taking into account common characteristics from those genera.
It can be speculated that Hessinodon would have a long, cylindrical body and a head with a short, blunt snout, large eyes, relatively massive jaws, and thick lips. For this reconstruction, the mouth anatomy follows Isistius more closely based on the possible ecology of Hessinodon (discussed below), while the body is more in line with Dalatias and other dalatiids, as Isistius is quite different from its relatives in this regard. The dorsal and pectoral fins of Isistius are proportionally small compared to its body, suggesting they are poor, or at least inactive, swimmers (Widder, 1998; de Figueiredo Petean & de Carvalho, 2018); it is impossible to determine whether Hessinodon was this specialized, so the reconstructed fins are largely based on Dalatias as a conservative approach. In the absence of tangible data, it is very likely that this reconstruction is at odds with the actual appearance of Hessinodon, but it serves as a suitable approximation that can be derived from its close relatives.

Precisely estimating the size of Hessinodon is also virtually impossible, but comparing the teeth size with those of I. brasiliensis provides a reasonable ballpark. Based on comparisons with several I. brasiliensis individuals (Welton, 1979; de Figueiredo Petean & de Carvalho, 2018), Hessinodon appears to be around 35 to 40 cm in length, similar in size to modern Isistius, which range from 30 cm to over 50 cm (Ebert, Fowler, & Dando, 2021). Using I. plutodus as a reference was eschewed here, as its proportionally massive teeth means that comparing it with Hessinodon – which more likely had proportionally smaller teeth, following I. brasiliensis and other dalatiids – would probably end up underestimating the latter’s length.
An interesting phenomenon that almost certainly contributed to the appearance of Hessinodon in life is bioluminescence. Virtually all extant dalatiids* have bioluminescence (Claes & Mallefet, 2009; Grace et al. 2019), with Dalatias being the largest known luminous vertebrate (Mallefet, Stevens, & Duchatelet, 2021). It is produced by photophores largely concentrated on the ventral side of the body (Hubbs, Iwai, & Matsubara, 1967; Seigel, 1978; Bass, Compagno, & Heemstra, 1986; Claes, Ho, & Mallefet, 2012; Claes et al. 2014; Grace et al. 2015; Stehmann, Van Oijen, & Kamminga, 2016; de Figueiredo Petean & de Carvalho, 2018; Grace et al. 2019; Mallefet, Stevens, & Duchatelet, 2021; Delroisse et al. 2021), which create a counterillumination. This trait is inferred to be ancestral, shared by the common ancestor of Dalatiidae, Etmopteridae, and Somniosidae (Straube et al. 2015). Considering all this, bioluminescence was most likely present in Hessinodon, visible on the ventral side and forming a counterillumination.
*The presence or absence of photophores has not yet been confirmed in Mollisquama parini, but more than likely has them based on its sister species M. mississippiensis.


The counterillumination created by this bioluminescence functions as camouflage for dalatiids, allowing them to obscure their silhouette in the water column (Claes et al. 2014; Duchatelet et al. 2021). This may have allowed them not only ambush prey more effectively, but also evade predators. While the unique luminescent pattern of I. brasiliensis, caused by the dark ‘collar’ (alternatively, the black band) between the gills and pectoral fins lacking photophores, was initially proposed to act as a lure for potential prey (Widder, 1998), there is a lack of data to support this hypothesis. Furthermore, this would be very unlikely to attract the large animals that it feeds from (Duchatelet et al. 2021). It may serve as a form of intraspecific recognition instead, much like the markings of etmopterids (Claes et al. 2014). This purpose would appear to be limited to this species; I. plutodus lacks the dark ‘collar’ despite having the same photophore distribution and diet (de Figueiredo Petean & de Carvalho, 2018). On the other hand, the bright luminosity of the tails of D. licha and Squaliolus aliae seem more likely to have a luring function (Duchatelet et al. 2021). Notably, Euprotomicroides zantedeschia uses bioluminescent fluid as a defense mechanism, being released from a pelvic pouch with secretory glands; Mollisquama also has pectoral pockets with glands that most likely had the same function (Grace et al. 2019; Duchatelet et al. 2021). This is a highly derived adaptation, and it is more likely that Hessinodon had bioluminescence functioning as camouflage, as with other dalatiids.
Paleoecology and Paleogeography
The strong similarities between their lower dentitions suggests that the diet of Hessinodon was much like that of Isistius. Although the ectoparasitic part of its lifestyle is what makes Isistius so widely known, analysis of stomach contents (Strasburg, 1963; Jahn & Haedrich, 1987; Murakami, Yoshida, & Yonezaki, 2018) and biochemical tracers from individuals near Hawaii (Carlisle et al. 2021) actually show that squids and small mesopelagic and epipelagic fish, like Ariomma and Cololabis, are major components of their diet, at least for I. brasiliensis. Notably, the squids appear to have been consumed whole rather than being scavenged, and impressively some were estimated to be roughly the same size as the sharks themselves! This is not to say that feeding from large marine animals are an insignificant part of their diet; although the importance of such prey to Isistius was overestimated (Carlisle et al. 2021), flesh plugs from fish and mammals still make up a good number of their stomach contents (Jahn & Haedrich, 1987; Murakami, Yoshida, & Yonezaki, 2018), and indeed the bite marks left on these animals are well-documented (for a comprehensive list of these reports, see Supporting Information 2 of de Figueiredo Petean & de Carvalho, 2018). These chunks are ripped out by biting into prey, creating an oral vacuum to hold on, then quickly twisting its body in a circular fashion (Shirai & Nakaya, 1992). Conversely, Isistius has been found in the stomachs of blue sharks (Prionace glauca; Soto & Mincarone, 2004; Stehmann & Kukuev, 2014), swordfish (Xiphias gladius; Soto & Mincarone, 2004; Young et al. 2006; Ruiz-Abierno, Rojas-Corzo, & Angulo-Valdés, 2016), and a Spanish mackerel (Scomberomorus sp.; Isouchi, 1970), showing that cookiecutter sharks do end up as prey of larger predators.
Using Isistius as a basis, the trophic ecology of Hessinodon can be speculated in the context of the Northumberland Formation. Cyrtobelus hornbyense, a tiny stem-decabrachian cephalopod, and the young of other animals, like large octobrachians and fish under 2 m in length, were likely viable smaller prey for Hessinodon. Assuming that part of its lifestyle was spent as a ectoparasite like Isistius, Hessinodon could have fed from a multitude of significantly larger fish, ranging from 70 cm long enchodontids and >1 m long Squalus to the massive 6 m long Proteothrinax ludvigseni and 7 m long Dykeius garethi, biting out chunks of flesh and leaving behind characteristic circular wounds in the process. One group of animals that Hessinodon possibly had trouble predating on are the coexisting mosasaurs, as their scales may have provided enough protection to withstand the bites and deter the sharks. On the flip side, the small size of Hessinodon probably made it easy prey of not just other fish, but also coexisting large coleoids like Enchoteuthis and Actinosepia.

Although the exact environmental preference and behaviour of Hessinodon cannot be known, it can be inferred from extant dalatiids. They are generally considered to be deep sea sharks, but occupy a variety of depths, being collected from near the ocean surface over deep water to depths exceeding 2 km (Ebert, Dando, & Fowler, 2021). These ranges can be explained for several species by vertical migrations from deeper to shallower waters at night (Hubbs, Iwai, & Matsubara, 1967; Seigel, 1978). Isistius brasiliensis in particular comes into contact with its large vertebrate prey through these movements (Le Boeuf & McCosker, 1987; Papastamatiou et al. 2010; Murakami et al. 2018; Carlisle et al. 2021; Menezes et al. 2022), creating opportunities to feed off them. It is possible Hessinodon also exhibited this behaviour, moving up and down the water column during different times of the day. This is plausibly supported, albeit weakly, by the depositional environment of the Northumberland Formation being a 100-300 m deep outer shelf (Jenkins et al. 2017), a rather shallow depth for extant dalatiids. The rarity of this taxon from this environment – out of the some 2,000 teeth collected from this formation over the years, only four of Hessinodon have been recorded (in addition, this specimen from Adam Anderson) – may suggest that it frequented deeper waters in the mesopelagic and bathypelagic zones, but also was capable of vertical migrations into shallower depths.
Being only found from the Northumberland Formation thus far, it is unknown how far Hessinodon may have ranged outside of the British Columbian Pacific, if it did at all. However, most extant dalatiids are known to have very wide ranges (Ebert, Dando, & Fowler, 2021), so it is quite likely Hessinodon did as well. Furthermore, almost all of the coexisting sharks from the Northumberland Formation have either been found in other roughly contemporaneous regions or have close relatives. Examples include the hexanchid Xampylodon dentatus, known elsewhere from Santonian Japan, Campanian-Maastrichtian USA, upper Campanian-lower Maastrichtian Angola, Maastrichtian New Zealand, and upper Maastrichtian Argentina (Cappetta, Morrison, & Adnet, 2021; Kanno et al. 2022 and references within); the chlamydoselachid Proteothrinax, known elsewhere from Santonian Japan, upper Campanian-lower Maastrichtian Angola, and Santonian-Campanian and Maastrichtian Antarctica (Goto, 2004; Cappetta, Morrison, & Adnet, 2021 and references within); and the fellow dalatiid Squaliodalatias, known elsewhere from lower Paleocene Denmark (Adolfssen & Ward, 2015), lower Eocene of France (Adnet, Cappetta, & Reynders, 2006), California, and Denmark (Welton, 2016), middle Eocene France (Adnet, Cappetta, & Reynders, 2008), and lower Miocene Slovakia (Underwood & Schlögl, 2013). This opens up the potential for finding the highly distinctive teeth of Hessinodon in other areas, and it is hoped that these prospective discoveries, alongside future material being potentially collected from the Northumberland Formation, will further elucidate this unique and strange taxon.
One thought on “Reconstructing Hessinodon wardi, the Cretaceous cookie cutter”