ISSN: 0973-7510
E-ISSN: 2581-690X
Dermatophytosis is a prevalent infection in tropical and subtropical countries, including India. This study aims to investigate the epidemiology and clinical aspects of chronic and recurrent dermatophytosis, identify the clinical isolates, and assess the effectiveness of different microscopy and staining methods for diagnosis. The objective of the study is to study the epidemiology and clinical aspects of chronic and recurrent dermatophytosis and to identify the clinical isolates. Attempts to improve the diagnostic outcome by implementing different microscopy and staining methods have also been performed. Adult patients with chronic and recurrent cases and positive direct microscopy were included, and clinical details were recorded. The mycological culture was performed. Fifteen isolates were selected randomly and subjected to 0.9% NaCl, Chicago sky blue staining, Calcofluor white staining, and Congo red staining in addition to the standard lactophenol cotton blue (LPCB) preparation. Among the 178 patients in the study, females (56.7%) and patients aged 25-45 (50%) were more frequently affected. Tinea corporis was the most common clinical type (89.32%). Recurrent cases (56.1%) were more prevalent than chronic cases (43.9%). Culture positivity was seen in 60.1%, with Trichophyton mentagrophytes being the most common isolate (86%). Among the staining methods used on 15 selected isolates, Lactophenol Cotton Blue (LPCB) was scored as the most effective, scoring 2.6 out of 3. The study revealed several clinical and epidemiological findings related to dermatophytosis in India, including a high degree of communicability, inadvertent therapies including topical steroid misuse, and a persistent nature requiring an extended duration of therapy. Trichophyton mentagrophytes was the most frequently isolated pathogen. Of the staining methods evaluated, Lactophenol Cotton Blue (LPCB) was found to be the most effective. The findings suggest a need for continued research into effective treatments and diagnostic methods for dermatophytosis in India.
Chronic Dermatophytosis, Recurrent Dermatophytosis, Mycological Diagnosis, Lactophenol Cotton Blue, Calcofluor White, Congo Red
Dermatophytosis is the most common fungal skin infection that often requires laboratory analysis to confirm and assess a mycological cure. These infections are encountered worldwide but are found prominently in tropical countries like India due to favorable climates.1 Direct microscopy with potassium hydroxide mount is routinely performed in many clinical settings. Mycological culture is sought to recognize the species.2 The most common procedure in clinical laboratories for identifying molds based on their distinctive morphological characteristics is still the microscopic examination of wet mounts. Although the traditional identification method of species, such as Lactophenol cotton blue (LPCB) preparations, is useful, more sensitive procedures are for rapid and ease identification.3 This study aimed to identify the common species causing dermatophytosis in the current scenario and evaluate the usefulness of other not so commonly performed methods in species identification on microscopy.
Study setting and study design
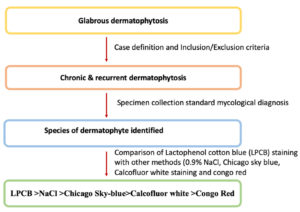
The study was undertaken in the Department of Dermatology and Microbiology of a tertiary care medical college, along with the help of a research laboratory of the university. Written informed consent was obtained from all participating patients considered for the study. Patients aged above 18 years with clinically suspected cases of chronic and recurrent dermatophytosis were screened by a dermatologist from February 2020 to January 2021. The flow chart showing the visual representation of the study’s outline is depicted in Figure 1.
Patient selection
The presence of glabrous tinea for a duration of six months or longer was considered as chronic dermatophytosis, and reoccurrence of the glabrous tinea after 4 weeks of stopping treatment was regarded as recurrent dermatophytosis.4 Detailed clinical and epidemiological data were taken, and the diagnosis was given. Skin scrapings for fungal structures on direct microscopy with potassium hydroxide mount were done, and patients with negative findings were excluded from the analysis. Positive scrapings were later subjected to mycological culture. Potassium hydroxide mount with direct microscopy was performed by standard technique.4,5
Laboratory methods
Direct microscopy
For direct microscopy, a drop of 10% KOH was added to the slide containing skin scrapings collected from the patients. To get rid of the extra KOH, a cover slip was put on top and gently pressed down. The slide was kept aside for 10 minutes until the digestion of the specimen occurred. Slides were microscopically evaluated for the presence of branching thread-like structures (hyphae) or beaded spherical structures (spores). When they were present, it was considered a positive test.5
Mycological culture
Skin scrapings were collected in sterile black chart papers. It was cultured in Sabouraud dextrose agar (SDA) medium with chloramphenicol (0.004%) and cycloheximide (0.05%) to prevent bacterial contamination. The plates were kept at 28°C for 4 to 5 weeks of incubation and were observed weekly for growth. If there was no growth, it was considered negative and discarded after four weeks. Examination with identification of the isolates was based on the colony character and microscopy. Lactophenol cotton blue (LPCB) staining was chosen to identify the microscopic characteristics of the vegetative structures. Tease mount preparation was performed from the growth, and one to two drops of the LPCB was added. It was observed under light and oil immersion microscopy.
A total of 15 isolates were subjected to 4 more different microscopic methods, viz., 0.9% NaCl, Chicago sky blue staining, Calcofluor white staining, and Congo red staining. The stains used in the study were prepared in house. Wet mount preparations for all the stains were prepared and examined under a microscope. A fluorescent cell imager was utilized for the last two methods. Photomicrographic or fluorescent images were collected and analyzed by two microbiologists who are not part of the study on a scale of 3 for ease of morphological identification. The results of the different methods were compared with that of the traditional methods. Details of these laboratory methods are given here.
0.9% NaCl
Normal saline is prepared for the identification of the dermatophytes. A drop of 0.9% saline is added to the slide, and the specimen is mounted on the slide, covered with a coverslip, and observed under the microscope.6
Chicago sky-blue staining
Chicago sky blue stain is a contrast stain. One drop of 1% Chicago sky blue stain and one drop of 10% KOH as a clearing agent were added to specimens mounted on slides and covered with a coverslip. All slides were then examined by light microscopy at ×100 and ×400 magnification.7
Calcofluor staining
One drop of Calcofluor white stain, comprising one g/L Calcofluor white (Sigma-Aldrich), was added to each specimen and mounted on a slide. Slides were left to stand for 10 mins and examined under fluorescence microscopy (Zoe fluorescent cell imager).8
Congo red staining
Congo red is a quantitative method used for the identification of dermatophytes. When seen under the fluorescent microscope, the fungal elements appear red on a pink or light orange in the background.9
Ethical approval
The study received approval from the institutional ethical committee with the reference number YEC-1/10/2020 dated 22-01-2020.
Statistical analysis
The data were entered into Microsoft Excel 2019 v16.0 (Microsoft, Redmond, WA, USA) and subsequently analysed using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA) software. A comprehensive descriptive analysis was performed to generate summary statistics, which were presented as frequencies and percentages for the sociodemographic characteristics. Pearson’s Chi-squared test was employed to assess associations between categorical variables, while Fisher’s exact test was used when one or more cells had an expected frequency of five or fewer. The level of significance was set at 5% (p-value ≤ 0.05) threshold.
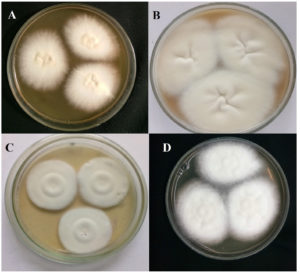
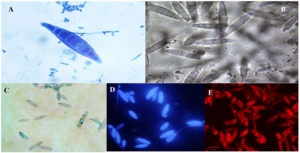
In the present study, the samples were collected for 12 months. A total of 178 patients were enrolled in the study after satisfying the inclusion and exclusion criteria. The sociodemographic and clinical characteristics of patients are shown in Table 1. Tinea corporis was the most common clinical type (159,89.32%), as depicted in Table 2. The distribution of clinical type among enrolled subjects with mycological culture results is depicted in Table 2. Patients aged 25-45 (89/178, 50%) were found to have the infection more frequently. The female ratio (101/178, 56.7%) was more than that of the male (77/178, 43.3%). Outdoor workers (49, 27.5%) were more frequently affected by the infection compared to people involved in other occupations. Family history of skin disease suggestive of tinea was present in 115 (64.6%) participants. Compared to the chronic cases (78, 43.8%), recurrent cases (100, 56.2%) were more prevalent. All the scrapings were positive for KOH mount as per the inclusion criteria. Culture positivity was seen in 107 (60.1%), with Trichophyton mentagrophytes (92, 86%) being the most common isolate (Table 3). This was followed by Trichophyton rubrum (7, 6.5%) and Nannizzia gypsea (6, 5.6%). Figure 2 shows the various cultural characteristics of macroscopy. Randomly selected 15 isolates were also subjected to five staining methods as mentioned in the methodology. According to the two microbiologists who evaluated microscopic images, LPCB was scored as the preeminent with a score of 2.6 out of 3 (Table 4). This was followed by NaCl (Score 2), Chicago sky blue (Score 1.8), Calcofluor white (score 1.7), and Congo red (Score 1.6). Figure 3 and Figure 4 depict the microscopic characteristics of the isolates.
Table (1):
Sociodemographic and clinical characteristics of study patients stratified by chronic and recurrent (N=178)
| Disease type | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chronic (N=78) | (n 43.8%) | Recurrent (N=100) | (n= 56.2%) | Total number (N) | % | Chi-square | P value | ||
| Gender | Male | 33 | 42.3 | 32 | 32 | 65 | 36.5 | 2.008 | 0.156 |
| Female | 45 | 57.6 | 68 | 68 | 113 | 63.4 | |||
| Age group | 18-25 | 26 | 33.3 | 25 | 25 | 51 | 28.6 | 2.429 | 0.488 |
| 26-45 | 33 | 42.3 | 47 | 47 | 80 | 44.9 | |||
| 46-59 | 17 | 21.7 | 22 | 22 | 39 | 21.9 | |||
| ³60 | 2 | 2.5 | 6 | 6 | 8 | 4.49 | |||
| Occupation | Outdoor working | 23 | 29.4 | 31 | 31 | 54 | 30.33 | 1.880 | 0.758 |
| Indoor working | 17 | 21.7 | 23 | 23 | 40 | 22.47 | |||
| Students | 13 | 16.6 | 20 | 20 | 33 | 18.5 | |||
| Homemakers | 10 | 12.8 | 07 | 07 | 17 | 9.5 | |||
| Others | 15 | 19.2 | 19 | 19 | 34 | 19.1 | |||
| Comorbidities | Diabetes | 12 | 15.3 | 5 | 5 | 17 | 9.5 | 12.106 | 0.033* |
| Hypertension | 5 | 6.4 | 8 | 8 | 13 | 7.3 | |||
| Cardiac disease | 1 | 1.28 | 6 | 6 | 07 | 3.9 | |||
| Acid-peptic disease | 0 | 0 | 5 | 5 | 5 | 2.8 | |||
| Others | 4 | 5.12 | 8 | 8 | 12 | 6.7 | |||
| None | 56 | 71.7 | 68 | 68 | 124 | 69.6 | |||
| Treatment history (past and current) | Topical antifungal alone | 12 | 15.3 | 06 | 06 | 18 | 10.1 | 8.674 | 0.069 |
| Topical antifungal + systemic antifungal | 28 | 35.8 | 38 | 38 | 66 | 37.07 | |||
| Topical corticosteroid (TCS) or TCS combination (TCSC cream | 23 | 29.4 | 32 | 32 | 55 | 30.8 | |||
| TCS/TCSC + systemic antifungal | 15 | 19.2 | 18 | 18 | 33 | 18.5 | |||
| No treatment | 0 | 0 | 06 | 06 | 06 | 3.3 | |||
*Statistically significant
Table (2):
Distribution of clinical types among enrolled subjects stratified by mycological culture results (N=178)
| Mycological culture | |||
|---|---|---|---|
| Clinical Types | Overall cases (N=178) | Positive 107 (60.2%) | Negative, 71 (39.8%) |
| Tinea corporis | 159 | 97 | 62 |
| Tinea cruris | 128 | 79 | 49 |
| Tinea faciei | 19 | 12 | 7 |
| Tinea pedis | 16 | 10 | 6 |
| Tinea manuum | 9 | 8 | 1 |
| Tinea unguium | 2 | 0 | 2 |
| Total events* | 333 | 206 | 127 |
*Some patients may have more than one type of infection
Table (3):
Culture positivity of the laboratory specimens
Details |
Number of specimens |
Percentage |
|---|---|---|
Total samples |
178 |
100 |
Dermatophytes |
107 [Trichophyton mentagrophytes (92. 86%), Trichophyton rubrum (7, 6.5%), Nannizzia gypsea (6,2, 5.6%), Microsporum canis (2,1.9%)] |
60.11 |
Other fungal isolates (Contaminants) |
40 |
22.47 |
Negative Culture |
31 |
17.41 |
Table (4):
Comparison of diagnostic utility of the different microscopic and staining methods in dermatophytosis (Total samples tested= 15*)
No |
Stains used |
Average score |
|---|---|---|
1 |
Lactophenol cotton blue |
2.6 |
2 |
Sodium chloride |
2 |
3 |
Chicago Sky Blue |
1.8 |
4 |
Calcofluor White |
1.7 |
5 |
Congo Red |
1.6 |
*Evaluated by two independent microbiologists with a scoring range of 0 to 3
Figure 2. (a) Trichophyton mentagrophytes colonies on Sabouraud Dextrose Agar; flat, creamy, and granular surface with reverse yellow-brown pigmentation. (b) Trichophyton rubrum colonies on Sabouraud Dextrose Agar; flat, creamy, and downy with reverse red pigmentation. (c) Microsporum canis on Sabouraud Dextrose Agar gives a white to yellowish colony with a coarsely fluffy, velvety/powdery texture. (d) Nannizzia gypsea colonies on Sabouraud Dextrose Agar; flat, creamy, and granular with reverse yellow-brown pigmentation.
Figure 3. Microscopy of Trichophyton mentagrophytes showing numerous club-shaped microconidia along with the spiral hyphae in five different staining methods using (A)Lactophenol cotton blue(B) 0.9% NaCl (C) Chicago sky blue(D) Trichophyton mentagrophytes was observed under the fluorescence microscope using calcofluor white stain was stained blue (E) Trichophyton mentagrophytes was observed under the fluorescence microscope using Congo red where the macroconidia were stained red.
Figure 4. Microscopy of Nannizzia gypsea showing spindle-shaped with 5-15 cells, thick walled often with terminal knob in five different staining methods using (A)Lactophenol cotton blue(B)0.9% NaCl (C) Chicago sky blue(D) Nannizzia gypsea was observed under the fluorescence microscope using calcofluor white stain was stained blue (E) Nannizzia gypsea was observed under the fluorescence microscope using Congo red where the macroconidia were stained red.
Dermatophytes are fungi that grow on skin, hair, and nail, causing infections commonly known as tinea.10 They are generally classified into three genera, Epidermophyton, Trichophyton, and Microsporum, although new genera like Nannizzia, Lophophyton, Paraphyton, and Arthroderma have been recognized recently.11 Tinea corporis is considered to be the most common condition caused by dermatophytes worldwide. Common dermatophytes that cause tinea corporis infections include Trichophyton rubrum and Trichophyton mentagrophytes. Generally, tissue invasion is confined to the cutaneous layer because of the inability of the fungi to penetrate deeper tissues; however, occasionally, subcutaneous invasion occurs.12
India has been experiencing a hyperendemic scenario, and hence precise identification of the agent is required for the purpose of epidemiology and to understand their sensitivity to antifungals.13 Several clinical and epidemiological features that have been described in the past one decade are different from the previously reported studies. A high rate of false-negative results for dermatophyte detection may commonly occur due to poor samples or laboratory techniques.14 The two standard methods for identifying dermatophytes in a laboratory setting are a direct demonstration of fungal elements with KOH mount and isolation of dermatophytes in vitro fungal culture. Before treating patients with any antifungals, confirming the clinical diagnosis with laboratory findings is desirable due to tremendous morphological deviations experienced in the current clinical scenario of dermatophytosis that may lead to misdiagnosis. The collection of samples also plays an important role in the identification of fungal species.15
Clinical features observed in our study have been observed by several studies in the recent literature. High incidences of chronic and recurrent infections, infection of family members, infection among young adults, and infection among outdoor workers seem to be the epidemiological factors constantly associated with the current scenario.16 It is not clear whether these findings are common for all dermatophyte species or to any specific species. Recent reports have documented the identification of a novel species, namely Trichophyton indotineae, which is purported to be the predominant causative agent in India, surpassing all other previously prevalent species. The study conducted by Uhrlarr et al. raises the hypothesis that these epidemiological findings may be attributable to specific virulence factors associated with this newly identified species.17
A total of 178 skin scrape samples from patients diagnosed with tinea corporis were included in this study. Among these samples, 101 (56.7%) were obtained from female patients, while 77 (43.3%) were obtained from male patients. The most common age group was found to be 25-45 (89, 50%). Direct microscopy was performed on all 178 (100%) samples, using KOH preparations, which are commonly utilized for the initial identification of dermatophytes due to their high sensitivity and specificity. However, it is important to note that KOH preparations have been associated with false-negative results ranging from 5% to 15%. This may be attributed to the limited visibility of small, scattered fungal material in the samples obtained. The accuracy of fungal identification through direct microscopy relies on the expertise of the observer.18-21 Direct microscopic examination of KOH-prepared material is a cost-effective and straightforward approach employed for diagnosing mycotic infections. However, it is accompanied by both advantages and disadvantages. To minimize the risk of laboratory contamination, confirmation through direct microscopy is often required for isolates grown on culture.22
In our study, the culture positivity rate for dermatophytosis was found to be 60.1%. This aligns with a previous study conducted at our institution in 2017, which reported a similar rate of 62.3%. Another study conducted at our centre, focusing on therapy-resistant dermatophytosis, revealed a slightly lower positivity rate of 42.7% in 2019. The literature encompasses several studies on dermatophytosis, with isolation rates ranging from approximately 50% to 60%.23,24 Our finding shows that Trichophyton mentagrophytes was the most common isolate (92, 86%), followed by Trichophyton rubrum (7, 6.5%). Similar findings were observed in the previous studies conducted in our institution and elsewhere.25-28 In the past, it was reported by several studies that the Trichophyton rubrum was the most common agent to be isolated between 2002 and 2011. Trichophyton mentagrophytes are undoubtedly emerging as the predominant pathogen responsible for glabrous dermatophytosis in India.
The identification of isolates through conventional methods, such as mycological culture, poses certain challenges. Macroscopic characteristics are not always useful in identifying the species, and hence the microscopic appearance of vegetative structures is the most frequently employed in conventional diagnosis. An exercise was conducted using 15 randomly selected isolates, and 4 more different staining/microscopy were conducted to rate these methods for future studies.29,30,6
There are different stains available for the identification of dermatophytes, but only some stains are routinely used in the lab for the identification of dermatophytes. Lactophenol cotton blue stain could be one of the most frequently employed staining methods for physicians and microbiologists to be used for identification compared to the other staining techniques. LPCB is characterized by its affordability, easy accessibility, straightforward preparation, and stability at room temperature. Additionally, it ensures the safety of laboratory personnel. Chicago sky blue is a contrast stain that helps to distinguish between hyphae and epithelial cells and is used with KOH as a clearing agent. However, this stain does not require any heating which is required as the clearing agent. When stained, fungal filaments appear distinct blue against a pale or purple background.20 The identification of dermatophytes can sometimes pose challenges due to their faint staining characteristics, which can lead to difficulties in accurate identification. Chicago sky blue is not routinely used in the identification of dermatophytes. In recent times usage of fluorochrome stains for the identification of dermatophytes has been witnessed. Calcofluor white stain (CW) and congo red (CR) stains were used in the present study for the identification of dermatophytes. CW is a non-specific fluorochrome that binds with cellulose and chitin in the cell walls of cellulose-containing organisms and facilitates the visualization of pathogenic elements, and CR stains the chitin of the fungi.21 CR can be more easily dissolved in SDS than KOH.0.9% Sodium chloride is also one of the staining methods used for the identification of dermatophytes. The utilization of this stain for dermatophyte identification in laboratories is relatively uncommon. However, this method offers the advantage of being time-efficient and relatively uncomplicated in its procedural requirements. Identifying different methods of staining can ease the burden of identifying the species and also from the false negative results obtained during the process. We found in our study that LPCB was able to stain all the fungal elements, macroconidia and microconidia. The vegetative structures and hyphae seem to have taken up the stain adequately, and the differentiation of the hyphae and other vegetative structures was seen. LPCB is considered one of the safest stains to use and is not time-consuming. In CW, the fungal elements emit blue fluorescence, and in CR, the filaments emit red fluorescence under the fluorescent microscope. The drawback associated with this staining is that the hyphae and the microconidia can get over-stained and emit a lot of radiation which might become difficult for identification; also, some of the fungal elements and spores are not clearly visible, and the results were satisfactory and would also potentially lead to false negative results. However, macroconidia were stained perfectly, and the cell wall of the fungi could be appreciated. Other method, 0.9% of Sodium chloride, had the appearance of that of KOH mount. This technique was able to highlight the morphological characteristics of both spores and hyphae, but the color contrast was not that clear to identify the fungal element when compared to that of the other stains.
According to the scoring done by two independent microbiologists, LPCB scored 2.6 out of 3, followed by NaCl at 2. Other methods Chicago Sky Blue at 1.8, Calcofluor White at 1.7, and Congo Red to be 1.6, seem to be almost equally rated. LPCB has been universally used, which is justified by our study. Fluorescent staining like CW and CR can be substituted to improve diagnostic acumen, but these methods cannot be recommended stand-alone.
Dermatophytosis of the glabrous skin continues to be a major health problem in India. Several epidemiological hallmarks, including a high degree of communicability, inadvertent therapies including topical steroid misuse, recalcitrant nature with a need for extended duration of therapy, and antifungal resistance, have been noted. Mycological diagnosis is required to understand the isolate prevailing in the region and to design tailor-made therapy. Microbiologists should be familiar with the microscopic morphology of dermatophytes. LPCB continues to remain the standard method of staining and seems to have the edge over other methods.
ACKNOWLEDGMENTS
The authors acknowledge the expertise provided by Dr. Suchitra Shenoy M, Department of Microbiology, Kasturba Medical College, Mangalore, India, and Dr. Asha Pai KB, Department of Microbiology, K S Hegde Medical Academy, Mangalore, India, in the evaluation of the microscopic images and scoring them. The authors also acknowledge Dr Ranajit Das, Division of Data Analytics, Bioinformatics and Structural Biology (DABS), Yenepoya Research Centre, Yenepoya (Deemed to be) University, Mangalore, India, for helping with the statistical analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MMS, VP, and NA conceptualized and designed the study. NA, MMS, and VP performed the literature review. MMS and NA collected the samples and recorded the data. VP and NA performed analyses. NA and MMS wrote the original draft. NA, MMS, and VP edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The data sets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
ETHICS STATEMENT
This study was approved by Yenepoya Ethics Committee – 1, Mangalore, India, with reference number YEC-1/10/2020, dated 22-01-2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J. 2016;7(2):77-86.
Crossref - Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(4):2-15.
Crossref - Shamly V, Kali A, Srirangaraj S, Umadevi S. Comparison of microscopic morphology of fungi using lactophenol cotton blue (LPCB), iodine glycerol, and congo red formaldehyde staining. J Clin Diagn Res. 2014;8(7):01-02 DL.
Crossref - Rengasamy M, Shenoy MM, Dogra S, et al. Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) Task Force against Recalcitrant Tinea (ITART) Consensus on the Management of Glabrous Tinea (INTACT). Indian Dermatol Online J. 2020;11(4):502-519.
Crossref - Bagga B, Vishwakarma P, Sharma S, Jospeh J, Mitra S, Mohamed A. Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian J Ophthalmol. 2022;70(2):542-545.
Crossref - Yu Y, Wolf AK, Thusek S, et al. Direct visualization of fungal burden in filamentous fungus-infected silkworms. J Fungi. 2021;7(2):136.
Crossref - Liu Z, Sheng P, Yang YP, et al. Comparison of modified Chicago sky blue stain and potassium hydroxide mount for the diagnosis of dermatomycoses and onychomycoses. J Microbiol Methods. 2015;112:21-23.
Crossref - Motamedi M, Lari MS, Pakshir K, Zomorodian K. Comparing real-time PCR and Calcofluor-white with conventional methods for rapid detection of dermatophytes: a cross-sectional study. J Microbiol Methods. 2019;161:84-86.
Crossref - Thakor R, Mistry H, Tapodhan K, Bariya H. Efficient biodegradation of Congo red dye using fungal consortium incorporated with Penicillium oxalicum and Aspergillus tubingensis. Folia Microbiol. 2022;67(1):33-43.
Crossref - Nenoff P, Kruger C, Schaller J, Ginter-Hanselmayer G, Schulte-Beerbuhl R, Tietz H-J. Mycology-an update part 2: dermatomycoses, clinical picture and diagnostics. J Dtsch Dermatol Ges. 2014;12(9):749 777.
Crossref - Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs in Context. 2020;9:5-6.
Crossref - Hsu S, Le EH, Khoshevis MR. Differential diagnosis of annular lesions. Am Fam Physician. 2001;64(2):289-296. PMID: 11476274.
- Das S, De A, Saha R, et al. The current Indian epidemic of dermatophytosis: a study on causative agents and sensitivity patterns. Indian J Dermatol. 2020;65(2):118-122.
Crossref - Lim SL, Lim CS. New contrast stain for the rapid diagnosis of pityriasis versicolor. Arch Dermatol. 2008;144(8):1058-1059.
Crossref - Shenoy MM, Teerthanath S, Karnaker VK, Girisha BS, Prasad MK, Pinto J. Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid-Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol. 2008;74(3):226-229.
Crossref - Shenoy MM, Rengasamy M, Dogra S, et al. A multicentric clinical and epidemiological study of chronic and recurrent dermatophytosis in India. Mycoses. 2022;65(1):13-23.
Crossref - Uhrlarr S, Verma B, Gräser Y, et al. Trichophyton indotineae-An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide-A Multidimensional Perspective. J Fungi. 2022;8(7):757.
Crossref - Sahai S, Mishra D. Change in spectrum of dermatophytes isolated from superficial mycoses cases: first report from Central India. Indian J Dermatol Venereol Leprol. 2011;77(3):335-6.
Crossref - Khan S, Singhal S, Mathur T, Upadhyay DJ, Rattan A. Antifungal susceptibility testing method for resource constrained laboratories. Indian J Med Microbiol.2006;24(3): 171-176.
Crossref - Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and non-dermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol.2003;149(2):296-305.
Crossref - Hindy N, Abiess AA. Isolation and identification of dermatophytes causing dermatophytosis in Hilla city, Iraq. Indian J Public Health Res Dev. 2019;10(10):2225-2230.
Crossref - Mourad B, Ismail M, Hawwam S, Msseha M, Hassan R. Evaluation of the efficacy of fluorescent staining and Chicago sky blue staining as methods for diagnosis of dermatophytosis in hair and nails. Clin Cosmet Investig Dermatol. 2019;12:751-758.
Crossref - Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J De rmatol Venereol Leprol. 2017;83:436-440.
Crossref - Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol. 2018;84(6):678-684.
Crossref - Amin N, Shenoy MM, Keekan KK, et al. Therapy:Resistant dermatophyte infection in India-clinico mycological study. APIK J Int Med. 2022;10(4):263-267.
Crossref - Saxena V, Shenoy M, Devrari J, Pai V, Agrawal V. A mycological study of tinea corporis:A changing epidemiological trend from Trichophyton rubrum to Trichophyton mentagrophytes in India. Indian J Dermatol Venereol Leprol. 2020;86(5):607.
Crossref - Tiwari S, Nanda M, Pattanaik S, Shivakumar GC, Sunila BS, Cicciù M, Minervini G. Analytical Study on Current Trends in the Clinico-Mycological Profile among Patients with Superficial Mycoses. J Clin Med. 2023; 12(9):3051.
Crossref - Bhatia VK, Sharma PC. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134.
Crossref - Lim CS, Lim SL. Practical tip: Chicago Sky Blue (CSB) stain can be added to the routine potassium hydroxide (KOH) wet-mount to provide a color contrast and facilitate the diagnosis of dermatomycoses. Dermatol Online J. 2011;17(8):11.
- Lodha N, Poojary SA. A novel contrast stain for the rapid diagnosis of pityriasis versicolor:A comparison of Chicago Sky Blue 6B stain, potassium hydroxide mount and culture. Indian J Dermatol. 2015;60(4):340.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.