Saccharomyces cerevisiae is a unicellular yeast cell that is found in the Kingdom fungi (singular: fungus). S. cerevisiae is found in the genus Saccharomyces and family Saccharomycetaceae. Morphologically, the cells of S. cerevisiae are ellipsoidal or cylindrical; and they can be propagated in the laboratory on simple mycological media such as Sabouraud dextrose agar (SDA) and potato dextrose agar (PDA).
The cells of S. cerevisiae are usually round to ovoid measuring 5–10 micrometers in diameter. Nutritionally, all the strains of S. cerevisiae can grow aerobically on simple carbohydrate source such as glucose, maltose and trehalose – which serves as their sole source of carbon and energy. They do not grow on media containing lactose and cellobiose. S. cerevisiae can grow aerobically and anaerobically on different carbohydrate-based media. They use urea, amino acids, peptides and ammonia (NH3) as their sole source of nitrogen.
Species of Saccharomyces also require some mineral elements like sulphur, phosphorus, iron, calcium and magnesium for optimum growth. S. cerevisiae reproduce asexually by the process of budding (Figure 1). S. cerevisiae is very instrumental in many industrial productions; and this has made it one of the most useful yeast since ancient times. Several strains of Saccharomyces cerevisiae is used in the production of beer, bread, wine, feed yeasts, food yeasts, industrial alcohols and spirits.
S. cerevisiae like Escherichia coli (a bacterium) is one of the most intensively studied eukaryotic model organisms in molecular and cell biology; and it has been applied in many biotechnological applications for the production of various economically important products (Table 1). The use of S. cerevisiae as a model for industrial productions and for molecular biology techniques especially in studying the physiology and metabolic processes of eukaryotic cells is largely attributed to its unique characteristics. S. cerevisiae has a small size and it has a small generation (doubling) time which allows it to grow rapidly on simple media.
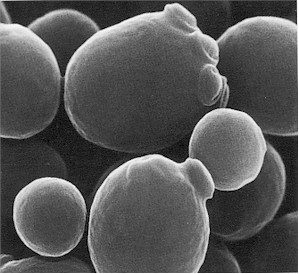
Table 1. Applications of various strains of Saccharomyces cerevisiae
| Yeast cells/strains | Products |
| Saccharomyces cerevisiae | Bread |
| S. cerevisiae | Ethanol |
| S. bayanus | Sparkling wine |
| S. cerevisiae | Top-fermenting beer |
| S. cerevisiae var. ellipsoids | Wine |
| S. uvarum | Bottom-fermenting beer |
| S. cerevisiae | Spirits |
| S. cerevisiae | Other yeast products |
| S. cerevisiae | Feed yeasts |
It has the ability to adapt to changing substrates and S. cerevisiae is amenable to genetic manipulations. S. cerevisiae has a high yielding capacity, and it produces large amount of carbondioxide (CO2) especially in flour dough. S. cerevisiae can be called several names depending on the type of industrial process it has been applied in.
Baker’s yeast, brewer’s yeast, top-fermenting yeast, ale yeast and budding yeast are some of the names that S. cerevisiae have been known for. For example, in bread production, S. cerevisiae is used in baking as a leavening agent – where it converts the fermentable sugars present in the dough into carbon dioxide gas. To the lay man, S. cerevisiae used in bread production is known as sodium bicarbonate (the leavening agent of bread).
The conversion of fermentable sugars in the dough by S. cerevisiae to CO2 gas causes the dough to rise as gas forms pockets or bubbles in the dough or bread. And when the bread is finally baked, the yeast cells dies and the air pockets created in the dough sets; and this gives the baked product a soft and spongy texture after production.
References
Bader F.G (1992). Evolution in fermentation facility design from antibiotics to recombinant proteins in Harnessing Biotechnology for the 21st century (eds. Ladisch, M.R. and Bose, A.) American Chemical Society, Washington DC. Pp. 228–231.
Nduka Okafor (2007). Modern industrial microbiology and biotechnology. First edition. Science Publishers, New Hampshire, USA.
Das H.K (2008). Textbook of Biotechnology. Third edition. Wiley-India ltd., New Delhi, India.
Latha C.D.S and Rao D.B (2007). Microbial Biotechnology. First edition. Discovery Publishing House (DPH), Darya Ganj, New Delhi, India.
Nester E.W, Anderson D.G, Roberts C.E and Nester M.T (2009). Microbiology: A Human Perspective. Sixth edition. McGraw-Hill Companies, Inc, New York, USA.
Steele D.B and Stowers M.D (1991). Techniques for the Selection of Industrially Important Microorganisms. Annual Review of Microbiology, 45:89-106.
Pelczar M.J Jr, Chan E.C.S, Krieg N.R (1993). Microbiology: Concepts and Applications. McGraw-Hill, USA.
Prescott L.M., Harley J.P and Klein D.A (2005). Microbiology. 6th ed. McGraw Hill Publishers, USA.
Steele D.B and Stowers M.D (1991). Techniques for the Selection of Industrially Important Microorganisms. Annual Review of Microbiology, 45:89-106.
Summers W.C (2000). History of microbiology. In Encyclopedia of microbiology, vol. 2, J. Lederberg, editor, 677–97. San Diego: Academic Press.
Talaro, Kathleen P (2005). Foundations in Microbiology. 5th edition. McGraw-Hill Companies Inc., New York, USA.
Thakur I.S (2010). Industrial Biotechnology: Problems and Remedies. First edition. I.K. International Pvt. Ltd. New Delhi, India.
