Abstract
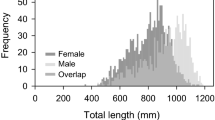
A population of the Hawaiian coral-reef goby, Asterropteryx semipunctata, was sampled over a 12-month period to determine basic demographics and reproductive parameters. The sexes did not differ in median length or weight, although the largest males were considerably longer and heavier than the largest females. Overall adult sex ratio was 1 : 1; monthly sex ratios did not differ from unity except in June, when there was a significant female bias. Minimum age at maturity (17.5–19 mm SL) was estimated to be 4.5–5 months after hatching. Nearly all fish over 22 mm SL were mature. Mature females were found in all months of the year, and females that showed evidence of recent or imminent spawning were collected in every month except December. Gonadal analyses indicated a peak in breeding during the summer (May–July) and minimal spawning during the winter (January–February). Between 20% and 30% of females showed evidence of having spawned within the 24-h period prior to collection; therefore, it was estimated that females spawned, on average, at least once every five days and perhaps as frequently as every three days. Mean batch fecundity was 708 eggs (± 418), and was not well predicted by standard length, body weight, or somatic condition. Relative batch fecundity (mean = 1.44 oocytes mg−1 somatic wet weight) varied seasonally, with higher values in spring and summer (April–July) than in fall and winter (September–January). Reproductive parameters are compared and contrasted with those of other gobiid fishes to elucidate general differences between temperate and tropical species.
Similar content being viewed by others
References cited
Bagenal, T.B. 1971. The interrelation of the size of fish eggs, the date of spawning and the production cycle. J. Fish Biol. 3: 207–219.
Charnov, E.L. & D. Berrigan. 1990. Dimensionless numbers and life history evolution: age of maturity versus the adult lifespan. Evol. Ecol. 4: 273–275.
Choat, J.H. 1982. Fish feeding and the structure of benthic communities in temperate waters. Ann. Rev. Ecol. Syst. 13: 423–449.
Clarke, T.A. 1987. Fecundity and spawning frequency of the Hawaiian anchovy or nehu, Encrasicholina purpurea. U.S. Fish. Bull. 85: 127–138.
Clarke, T.A. 1989. Seasonal differences in spawning, egg size, and early developmental time of the Hawaiian anchovy or nehu, Encrasicholina purpurea. U.S. Fish. Bull. 87: 593–600.
Clarke, T.A. & L.A. Privitera. 1995. Reproductive biology of two Hawaiian pelagic carangid fishes, the bigeye scad, Selar crumenophthalmus, and the round scad, Decapturus macarellus. Bull. Mar. Sci. 56: 33–47.
Cole, K.S. 1990. Patterns of gonad structure in hermaphroditic gobies (Pisces: Gobiidae). Env. Biol. Fish. 28: 125–142.
Colin, P.L. 1975. Neon gobies. T.F.H. Publications, Neptune City. 304 pp.
Conn, C.H. & D.L. Bechler. 1996. Reproductive strategies in a population of Gobiosoma bosci (Osteiechthyes: Gobiidae) with slowand fast maturing individuals. Gulf Research Reports 9(3): 177–182.
Dahlberg, M.D. & J.C. Conyers. 1973. An ecological study of Gobiosoma bosci and G. ginsburgi (Pisces, Gobiidae) on the Georgia coast. U.S. Fish. Bull. 71: 279–287.
Daoulas, C. & A.N. Economou. 1986. Seasonal variation of egg size in the sardine, Sardina pilchardusWalb., of the Saronikos Gulf: causes and a probable explanation. J. Fish Biol. 28: 449–457.
De Martini, E.E. 1991. Annual variations in fecundity, egg size, and the gonadal and somatic conditions of queenfish Seriphus politus (Sciaenidae). U.S. Fish. Bull. 89: 9–18.
De Martini, E.E. & M.E. Anderson. 1978. Comparative survivorship and life-history of painted greenling (Oxylebius pictus) in Puget Sound, Washington and Monterey Bay, California. Env. Biol. Fish. 5: 33–47.
De Martini, E.E. & R.K. Fountain. 1981. Ovarian cycling and batch fecundity in the queenfish, Seriphus politus: attributes representative of serial spawning fishes. U.S. Fish. Bull. 79: 547–560.
De Vlaming, V.L. 1972. Reproductive cycling in the estuarine gobiid fish, Gillichthys mirabilis. Copeia 1972: 278–291.
Dotu, Y. & S. Mito. 1963. The bionomics and life history of the eleotrid fish Asterropteryx semipunctatus Rüppell. Bulletin of the Faculty of Fisheries, Nagasaki Univ. 15: 10–16 (in Japanese).
Dotu, Y. & H. Tsukahara. 1964. The life history of the eleotrid fish, Mogurnda obscura (Temminck et Schlegel). Bulletin of the Japanese Society of Scientific Fisheries 30: 335–342 (in Japanese).
Ebeling, A.W & M.A. Hixon. 1991. Tropical and temperate reef fishes: comparison of community structures. pp. 509–563. In: P.F. Sale (ed.) The Ecology of Fishes on Coral Reefs, Academic Press, San Diego.
Economou, A.N., C. Daoulas, T. Psarras & R. Barbieri-Tseliki. 1994. Further data on the reproduction and development of Knipowitschia caucasica (Gobiidae). J. Fish Biol. 45: 360–362.
Fouda, M.M., M.Y. Hanna & F.M. Fouda. 1993. Reproductive biology of a Red Sea goby, Silhouettea aegyptia, and a Mediterranean goby, Pomatoschistus marmoratus, in Lake Timsah, Suez Canal. J. Fish Biol. 43: 139–151.
Gibson, R.N. 1967. The use of the anesthetic quinaldine in fish ecology. J. Anim. Ecol. 36: 295–301.
Gibson, R.N. 1970. Observations on the biology of the giant goby Gobius cobitis Pallas. J. Fish Biol. 2: 281–288.
Gibson, R.N. & I.A. Ezzi. 1978. The biology of a Scottish population of Fries’ goby, Lesueurigobius friesii. J. Fish Biol. 12: 371–389.
Gibson, R.N. & I.A. Ezzi. 1981. The biology of the Norway goby, Pomatoschistus norvegicus (Collett), on the west coast of Scotland. J. Fish Biol. 19: 697–714.
Grossman, G.D. 1979. Demographic characteristics of an intertidal bay goby (Lepidogobius lepidus). Env. Biol. Fish. 4: 207–218.
Ha, P.Y. & R.A. Kinzie. 1996. Reproductive biology of Awaous guamensis, an amphidromous Hawaiian goby. Env. Biol. Fish. 45: 383–396.
Healey, M.C. 1971a. Gonad development and fecundity of the sand goby, Gobius minutus Pallas. Trans. Amer. Fish. Soc. 100: 520–526.
Healey, M.C. 1971b. The distribution and abundance of sand gobies, Gobius minutus, in the Ythan estuary. J. Zool., Lond. 163: 177–229.
Healey, M.C. 1972. On the population ecology of the common goby in the Ythan estuary. J. Nat. Hist. 6: 133–145.
Hesthagen, I.H. 1977. Migrations, breeding, and growth in Pomatoschistus minutus (Pallas) (Pisces, Gobiidae) in Oslofjorden, Norway. Sarsia 63: 17–26.
Hislop, J.R.G. 1975. The breeding and growth of whiting, Merlangius merlangus in captivity. Journal du Conseil 36: 119–127.
Imai, C. & S. Tanaka. 1987. Effect of sea temperature on egg size of Japanese anchovy. Bull. Japan Soc. Sci. Fish. 53: 2169–2178.
Kamler, E. 1992. Early life history of fish: an energetics approach. Chapman & Hall, London. 267 pp.
Kinzie, R.A. 1993. Reproductive biology of an endemic, amphidromous goby Lentipes concolor in Hawaiian streams. Env. Biol. Fish. 37: 257–268.
Kuwamura, T., Y. Yugo & Y. Nakashima. 1994. Population dynamics of goby Paragobiodon echinocephalus and host coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 103: 17–23.
Lahnsteiner, F., M. Siewald, R.A. Patzner & E.A. Ferrero. 1992. The seminal vesicles of the male grass goby, Zosterisessor ophiocephalus (Teleostei: Gobiidae). Zoomorphology 111: 239–248.
Lassig, B. 1976. Field observations on the reproductive behavior of Paragobiodon spp. (Osteichthyes: Gobiidae) at Heron Island, Great Barrier Reef. Mar. Behav. Physiol. 3: 283–293.
Lowe-McConnell, R.H. 1979. Ecological aspects of seasonality in fishes of tropical waters. Symp. Zool. Soc. Lond. 44: 219–241.
Lugli, M., L. Bobbio, P. Torricelli & G. Gandolfi. 1992. Breeding ecology and male spawning success in two hill-stream populations of the freshwater goby, Padogobius martensi. Env. Biol. Fish. 35: 37–48.
Maciolek, J.A. 1977. Taxonomic status of Hawaiian Lentipes, a diadromous goby, with notes on its biology and distribution. Pac. Sci. 31: 355–362.
Manacop, P.R. 1953. The life history and habits of the goby Sicyopterus extraneus Herre (anga) Gobiidae with an account of the goby-fry fishery of Cagayan River, Oriental Misamis. Philippine J. Fisheries 2: 1–55.
Marconato, A., A. Bisazza & G. Marin. 1989. Correlates of male reproductive success in Padagobius martensi (Gobiidae). J. Fish Biol. 34: 889–899.
Marconato, A., M.B. Rasotto & C. Mazzoldi. 1996. On the mechanism of sperm release in three gobiid fishes (Teleostei: Gobiidae). Env. Biol. Fish. 46: 321–327.
Marsh, E. 1984. Egg size variation in central Texas populations of Etheostoma spectabile (Pisces: Percidae). Copeia 1984: 291–301.
Mashiko, K. 1976. Ecological study on breeding of an eleotrid goby, Odontobutis obscurus (Temminck et Schlegel), under rearing conditions. Japan J. Ecol. 26: 91–100.
McGinley, M.A. & E.L. Charnov. 1988. Multiple resources and the optimal balance between size and number of offspring. Evol. Ecol. 2: 77–84.
Miller, P.J. 1961. Age, growth and reproduction of the rock goby, Gobius paganellus L., in the Isle of Man. J. Mar. Biol. Ass. U.K. 41: 737–769.
Miller, P.J. 1975. Age-structure and life-span in the Common goby, Pomatoschistus microps. J. Zool., Lond. 177: 425–448.
Miller, P.J. 1984. The tokology of gobioid fishes. pp. 119–152. In: G.W. Potts & R.J. Wootton (ed.) Fish Reproduction: Strategies and Tactics, Academic Press, London.
Moreira, F., J.L. Costa, P.R. Almeida, C. Assis & M.J. Costa. 1991. Age determination in Pomatoschistus minutus (Pallas) and Pomatoschistus microps (Krøyer) (Pisces: Gobiidae) from the upper Tagus estuary, Portugal. J. Fish Biol. 39: 433–440.
Myers, R.F. 1999. Micronesian reef fishes. Coral Graphics, Barrigada. 330 pp.
Nash, R.D.M. 1982. The biology of Fries’ goby, Lesueurigobius friesii (Malm), in the Firth of Clyde, Scotland, and a comparison with other stocks. J. Fish Biol. 21: 69–85.
Nelson, J.S. 1994. Fishes of the world, 3rd ed. John Wiley & Sons, New York. 600 pp.
Parker, G.A. & M. Begon. 1986. Optimal egg size and clutch size: effects of environment and maternal phenotype. Amer. Nat. 128: 573–592.
Privitera, L.A. 2001. Characteristics of egg and larval production in captive bluespotted gobies. J. Fish Biol. 58: 1211–1220.
Provancha, M.J. & C.R. Hall. 1991. Ecology and life history of the clown goby inhabiting the upper Banana River, Cape Canaveral, Florida. Env. Biol. Fish. 31: 41–54.
Ragimov, D.B. 1987. Reproduction of small goby species (Gobiidae) of the Caspian Sea. J. Ichthyol. 27: 58–65.
Randall, J.E. 1996. Caribbean reef fishes. T.F.H. Publications, Neptune City. 368 pp.
Randall, J.E., G.R. Allen & R.C. Steene. 1990. Fishes of the Great Barrier Reef and Coral Sea. University of Hawaii Press, Honolulu. 507 pp.
Reavis, R.H. 1997. The natural history of a monogamous coralreef fish, Valenciennea strigata (Gobiidae): 2. behavior, mate fidelity and reproductive success. Env. Biol. Fish. 49: 247–257.
Roff, D.A. 1984. The evolution of life history parameters in teleosts. Can. J. Fish. Aquat. Sci. 41: 989–1000.
Rogers, S.I. 1989. Seasonal variations in fecundity and egg size of the common goby, Pomatoschistus microps. J. Mar. Biol. Ass. U.K. 69: 535–543.
Sakai, Y. 1996. Fecundity of female angelfish, Centropyge ferrugatus, independent of body size: field collection of spawned eggs. Ichthyological Research 43: 186–189.
Sawara, Y. 1978. Ecological studies on the common freshwater goby, Rhinogobius brunneus, especially on the growth, food habits and feeding activity. J. Fac. Sci. Univ. Tokyo, Section IV 14: 201–236.
Shafer, D.J. 1998. Early life history, growth and settlement dynamics of a tropical reef fish (Gobiidae: Bathygobius coalitus). Ph.D. Dissertation, Department of Zoology, University of Hawaii, Honolulu. 156 pp.
Shafer, D.J. 2000. Evaluation of periodic and aperiodic otolith structure and somatic-otolith scaling for use in reconstruction of the early life history of a tropical marine goby, Bathygobius coalitus. Mar. Ecol. Prog. Ser. 199: 217–229.
Smith, C.C. & S.D. Fretwell. 1974. The optimal balance between size and number of offspring. Amer. Nat. 108: 499–506.
Sponaugle, S. & R.K. Cowen. 1994. Larval durations and recruitment patterns of two Caribbean gobies (Gobiidae): contrasting early life histories in demersal spawners. Mar. Biol. 120: 133–143.
Springate, J.R.C., N.R. Bromage & P.R.T. Cumaranatunga. 1985. The effects of different ration on fecundity and egg quality in the rainbowtrout (Salmo gairdneri). pp. 371–393. In: C.B. Cowley, A.M. Mackie & J.G. Bell (ed.) Nutrition and Feeding in Fish, Academic Press, London.
Sunobe, T. & A. Nakazono. 1995. Embryonic development and larvae of genus Eviota (Pisces: Gobiidae) II. Description of seven species. Nat. Hist. Res. 3(2): 152–159.
Swenson, R.O. 1999. The ecology, behavior, and conservation of the tide-water goby, Eucyclogobius newberryi. Env. Biol. Fish. 55: 99–114.
Swift, C.C., J.L. Nelson, C. Maslow & T. Stein. 1989. Biology and distribution of the tidewater goby, Eucyclogobius newberryi (Pisces: Gobiidae) of California. Contrib. in Science 404, Natural History Museum of Los Angeles County, Los Angeles. 19 pp.
Tanasichuk, R.W. & D.M. Ware. 1987. Influence of interannual variations in winter sea temperature on fecundity and egg size in Pacific herring (Clupea harengus pallasi). Can. J. Fish. Aquat. Sci. 44: 1485–1495.
Thresher, R.E. 1984. Reproduction in reef fishes. T.F.H. Publications, Neptune City. 399 pp.
Townshend, T.J. & R.J. Wootton. 1984. Effects of food supply on the reproduction of the convict cichlid, Cichlasoma nigrofasciatum. J. Fish Biol. 24: 91–104.
Troitskiy, S.K. & Y.P. Tsunikova. 1983. Biology of the “monkey goby”, Neogobius fluviatilis (Gobiidae), in theKuban Lagoons. J. Ichthyol. 23: 39–45.
Vesey, G. & T.E. Langford. 1985. The biology of the black goby, Gobius niger L. in an English south-coast bay. J. Fish Biol. 27: 417–429.
von Bertalanffy, L. 1957. Quantitative laws in metabolism and growth. Quart. Rev. Biol. 32: 217–231.
Way, C.M., A.J. Burky, J.M. Harding, S. Hau & W.K.L.C. Puleloa. 1998. Reproductive biology of the endemic goby, Lentipes concolor, from Makamaka'ole Stream, Maui and Waikolu Stream, Moloka'i. Env. Biol. Fish. 51: 53–65.
Wiley, J.W. 1973. Life history of the western North American goby, Coryphopterus nicholsi (Bean). San Diego Soc. Nat. Hist. Trans. 17(14): 187–208.
Wiley, J.W. 1976. Life histories and systematics of the western North American gobies Lythrypnus dalli (Gilbert) and Lythrypnus zebra (Gilbert).). San Diego Soc. Nat. Hist. Trans. 18(10): 169–184.
Wootton, R.J. 1973. The effect of size of food ration on egg production in the female three-spined stickleback, Gasterosteus aculeatus L. J. Fish Biol. 5: 89–96.
Wootton, R.J. 1979. Energy costs of egg production and environmental determinants of fecundity in teleost fishes. Symp. Zool. Soc. Lond. 44: 133–159.
Wootton, R.J. 1998. Ecology of teleost fishes. Chapman & Hall, London. 392 pp.
Zar, J.H. 1996. Biostatistical analysis, 3rd ed. Prentice-Hall, Upper Saddle River. 662 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Privitera, L.A. Reproductive Biology of the Coral-reef Goby, Asterropteryx Semipunctata, in Kaneohe Bay, Hawaii. Environmental Biology of Fishes 65, 289–310 (2002). https://doi.org/10.1023/A:1020594900372
Issue Date:
DOI: https://doi.org/10.1023/A:1020594900372