Abstract
Variability over time in morphology and architecture of burrows excavated by Ocypode rotundata was studied on Salakh beach, Qeshm Island, Persian Gulf. According to our results, O. rotundata excavated single Shafts, J-shaped, Y-shaped, spiral and complex burrows, with spiral and complex burrows occurring only in adult crabs. The results showed that mating and reproduction behaviour of O. rotundata mainly occurred in the upper foreshore zone. Based on carapace width data and sex, percentage of J-shaped and Y-shaped burrow were more in female young crabs compared to males. Whilst, percentage of constructed single Shaft, J-shaped burrows were more frequent in male adult crabs compared to females. On the other hand, created complex burrow was observed just in male adult crabs. Also, male crabs created sand pyramid mounds seaward in front of the burrows. Four types of sand disposal behaviour were observed: knocking, throwing, slamming (only males) and stacking (only males). Results indicated that O. rotundata constructed burrows with any shape in all types of sediment, from the backshore to the foreshore. Sand moisture decreased with distance from the sea, which was associated with an increase in burrow depth. Temporal variation had significant impact on burrow slope, so that the entrance branch of all burrow shapes was clearly steeper during the reproduction period in contrast to the same burrows created in the non-reproduction period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ghost crabs are among the most common burrowing organisms on sandy beaches from the tropics to temperate latitudes (Lucrezi and Schlacher 2014; Jones 1972; Ma et al. 2019; Wong et al. 2012). These crabs use self-constructed burrows for shelter (Christoffers 1986; Kristensen et al. 2012), mating (Linsenmair 1967; Hughes 1973), egg development (Haley 1973), molting (Christoffers 1986), sex-specific signaling (Linsenmair 1967; Hartnoll 1969) and feeding (Crane 1941; Robertson and Pfeiffer 1982; Trott 1987). In addition, the tunnel diameter and depth is sufficiently small to prevent deformation and collapse of their burrows (Shinoda et al. 2019). With the exception of five or six zoea stages, which are planktonic (Díaz and Costlow 1972; McDermott 2009; Jiang et al. 2014), most ghost crabs spend their life in their self-constructed burrow. In this regard, digging activity of ghost crabs has important bioturbation effects on sediment characteristics, biogeochemistry and substrate (Schlacher et al. 2011). Thus, the burrowing behaviour of these crabs leads to improving aeration, substrate oxidation–reduction potential, and increases the complexity of the substrate (Chan et al. 2006; Lucrezi and Schlacher 2014).
Many studies have done on burrows of Ocypode crabs in recent years which have investigated morphological characteristics, distribution and architecture of their burrows within the specified time of the year (e.g. on O. ryderi (Vannini 1980), on O. saratan (Ali-Adnan 1985; Eshky 1985), on O. gaudichaudii (Schober and Christy 1993), on O. ceratophthalama (Chakrabarti 1981; Brooke 1981; Chan et al. 2006; Seike and Nara 2008; Lim et al. 2011), on O. sinensis (Seike and Nara 2008), on O. quadrata (McDermott 2009; Branco et al. 2010; Correa et al. 2014), on O. macrocera (Haque and Choudhury 2014)).
To date, two species of ghost crab have previously been reported on Iranian sandy beaches, namely, O. rotundata (Naderloo et al. 2015( and O. sinensis)Naderi et al. 2018a). Our target species, O. rotundata is commonly found in sandy beaches along the East coast of the Arabian Peninsula (Oman) to the west coast of India (Mumbai), including the Persian Gulf (Iran, Kuwait, Saudi Arabia, Bahrain, U.A.E.) (Naderloo and Türkay 2012; Sakai and Türkay 2013).This crab species has a life span of four years and can reach sizes of up to 60.02 mm carapace width (Naderi et al. 2019), with the main breeding season from March to October, e.g., in the Qeshm Island (Naderi et al. 2018b). Despite this considerable distribution range, few works have been conducted on the biology of O. rotundata (in Chabahar Bay, Najafi 2014; in Jask harbour, Zahedi et al. 2014, in Qeshm Island, Naderi et al. 2018a and 2019).
Detailed studies on the burrowing activity of these species are scarce. Burrows constructed by O. rotundata included Y-shaped, J-shaped, single Shaft, anchor, and spiral shapes (Zahedi 2014; Naderi and Pishehvarzad 2019). According to mentioned contents and dependence of O. rotundata on sandy beaches, this study will focus on the influence of environmental conditions (organic matter content of sediment, substrate moisture percentage, sand granulometry) and temporal variability (reproduction and non-reproduction period) on the shape and morphology of burrow construction of O. rotundata on foreshore and backshore of sandy beaches in Qeshm Island. Such studies could be important from point ichnology. So that, exegesis of the burrowing behaviour can help to better commentaries of the depositional conditions which ichnology is greatly applied. Also, study of trace fossils are useful as indicators of past sea-level position (Curran and White 1991) that it would first be necessary to do detailed morphological analyses of burrow types on sandy beaches of the world. On the other hand, our results will provide relevant information on the key features of a sensitive species inhabitant of Iranian sandy beaches; this could help in the design of conservation strategies to protect and manage a key coastal habitat, where human pressure and climate change will jeopardize its ecological values over the next few decades.
Methods
Study Area
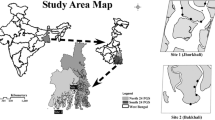
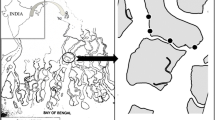
This study was conducted during the reproduction period of the ghost crab O.rotundata (April to September) and during the non-reproduction period (October to March) in Salakh sandy beach, Qeshm Island, Iran (Fig. 1) in February 2015, May, August and November 2016.
Based on topographic features, three areas were demarcated for Salakh beach which were foreshore, backshore, and dune zones. In this study, burrows were investigated on two zones: foreshore and backshore. The foreshore had a relatively flat surface with a seaward slope of 5°. The backshore was also flat, with a slope 2° landward. The dune zone was covered by vegetation, e.g., Halopayrum macronatum and Salsola vermiculata. Burrow distribution of O. rotundata was observed from the upper foreshore to the lower dune zone.
Experimental Design
The burrows of O. rotundata were distributed in 4000 m2 of sandy beach that along four transects (10 m width, positioned perpendicularly to sea, 10 m length), galleries with different diameters were chosen randomly; next, gypsum slurry (2:1 volume ratio of gypsum to water) was poured into the holes to obtain molds of the galleries` morphology. The distance of each burrow to the shoreline was noted. Crabs emerged from the burrows when the slurry poured and kept in plastic bags separately and labelled for further analysis. When the gypsum is set, the casts were carefully excavated by hand. The parameters of burrow morphology were measured, which included burrow opening diameter, length, depth, horizontal length and α and β angles (Fig. 2A, B). Out of the 106 burrows that were cast, 100 shapes were completed (56 burrows in non-reproduction period and 44 burrows in reproduction period), which were categorized into nine morphologies, as follows: single Shaft burrows (Fig. 3A), J-shaped (Fig. 3B), Y-shaped with short branch (Fig. 3C), Y-shaped (Fig. 3D, E), spiral burrow (Fig. 3F, G), complex burrows (Fig. 3H-J), J-shaped with extra branch (Fig. 3K), Y-shaped with an extra branch and anchor-shaped (Fig. 3L) and (Fig. 4). Regardless of this classification, the number of observed Y-shaped burrows with short branch, anchor-shaped, J-shaped with extra branch, Y-shaped with extra branch were very low, and thus were considered to be in the category of Y-shaped, complex burrows, that are J-shaped and Y-shaped, respectively. The carapace width and length of each crab were measured using vernier calipers, then weighed (analytical balance, ± 0.01 g, AND, EK-300i, Japan) and sexed according to abdomen, gonopod and pleopod morphology. According to size at the onset of maturity (Naderi et al. 2018b), all collected crabs were divided into two size classes of juvenile (mean: 25.52 ± 9.39 of carapace width) and adults crabs (mean: 44.55 ± 3.88 of carapace width).
Sediment samples with three repetitions (500 g) were collected at 0–50 cm and 50–100 cm depth intervals which used for analyzing the vertical differences in sediment composition.
The organic matter content of the sediment was calculated by loss of weight on ignition at 500 °C. To do this, three 10 g subsamples were placed in porcelain capsules and incinerated at 500 °C for 3 h (Mantelatto and Fransozo 1997).
where OM is organic matter content, W1 is Weight of tin (g), W2 is Weight of dry sediment + tin (g) and W3 is Weight of sediment after ignition + tin (g).
For calculating sediment moisture percentage, three 10 g subsamples were weighed and then dried at 105 °C in oven until a constant weight was attained. The following formula used to calculating sediment moisture percentage:
where MC is sediment moisture percentage, W1 is Weight of tin (g), W2 is Weight of moist sediment + tin (g) and W3 is Weight of dried sediment + tin (g).
For the study of sand granulometry, sand samples (100 g) were taken from a depth of 0–50 cm and 50–100 cm in the last burrow observed both seaward and landward. Seven grades of sand were obtained by dry sieving, as follows: Coarse silt (31–62 µm), very fine sand (62–125 µm), fine sand (125–250 µm), medium sand (250–500 µm), coarse sand (500–1,000 µm), very coarse sand (1–2 mm) and granules (2–4 mm) (Buchanan 1984).
For determination of sediment pH, three 70 g subsamples were placed into the glass beaker, added 70 cc distilled water for 24 h, and then pH of sediment was obtained by pH meter (Metrohm, 827, Switzerland).
GRADISTAT softwar (Version 4.0) was used to determine grain size (Blott and Pye 2001).
Statistical Analyses
Pearson correlation coefficient was estimated to determine the relationship between burrow opening diameter and carapace width. t-test was also performed to compare between length of primary and secondary branch of Y-shaped burrows. The data were analyzed by using statistical software R version 3.5.0. The proportions of constructed burrows were compared by multinomial proportions analysis.
Results
Overall, 100 shapes were completed. On the other hand, 30 crabs emerged from the burrows were collected (17 females and 13 males). Relationship between carapace width and burrow diameter (Pearson’s correlation coefficient = 0.57, t = 3.48, df = 25, P = 0.001), burrow depth (Pearson’s correlation coefficient = 0.37, t = 2.13, df = 28, P = 0.04), were positively correlated (Fig. 5A, B). Relationship between carapace width and horizontal length of Y shaped burrow (Pearson’s correlation coefficient = 0.57, t = 1.7, df = 6, P = 0.13), α angle (Pearson’s correlation coefficient = 0.03, t = 0.03, df = 26, P = 0.96) were uncorrelated which were showed in Fig. 5C, D. Also, the scatterplot and the regression line of carapace width against ratio of burrow diameter/carapace was showed in Fig. 6. There was not any significant correlation between carapace width and ratio of burrow diameter/carapace (Pearson’s correlation coefficient = 0.27, t = -1.48, df = 26, P = 0.14). According to results, constructed single Shaft burrows by host crabs (28.76 ± 11.04 mm of carapace width) had a mean opening diameter of 28.08 mm and depth of 44.14 mm. Spiral burrows had a mean opening diameter of 31.05 mm and were produced by crabs with mean carapace width of 37.36 mm which had longer depth with mean of 67.75 mm than other three types of burrow. J-shaped burrow with mean opening diameter of 32.14 mm were produced by crabs with mean carapace width of 32.22 mm. Y-shaped burrow had the smallest opening diameter with a mean depth of 43.87 mm which had constructed by crabs with mean carapace width of 26.6 mm. The single Shaft entrance was more inclined than other burrows (Fig. 5E). Only collected male crab from complex burrow had 48.4 mm and 42.8 mm width and length of carapace respectively which made burrow with opening diameter of 60.8 mm and depth of 37 mm.
Regression relationship between carapace width and A burrow diameter; B burrow depth. Spot plot of carapace width and A horizontal length (just in Y shaped burrow); D α angle. E The variations of carapace width of crabs, opening diameter, depth and inclination angle among the J-shaped, Y-shaped, spiral and single tube burrows in Ocypode rotundata
Based on carapace width, percentage of J-shaped and Y-shaped burrow were more in female young crabs (Fig. 7A). Whilst, percentage of constructed single Shaft, J-shaped burrows were more frequent in male adult crabs. Also, created complex burrow was observed just in male adult crabs (Fig. 7B).
Y shaped burrow was had most frequency (22 burrows) in comparison to others. On the other hand, frequency of Y (19 burrows), J-shaped (16 burrows) and spiral (11 burrows) were more observed in non-reproduction period whilst most number of single Shaft burrows (14 burrows) were collected in reproduction period. Furthermore, percentage of complex burrow was significantly low (P < 0.05). Conversely, there was not observed significant difference between other burrows (Table 1). Also, results showed that percentage of Y shaped and complex burrow was significantly lower and higher in reproduction period respectively. Moreover, there was not any significant difference between other constructed burrows in reproduction and non-reproduction period (Table 2).
Single Shaft, J-shaped, Y-shaped and spiral burrows were observed in months of February, May, August and November, whilst complex burrows occurred only in May and August (reproduction period) (Fig. 4).
Figure 8 shows Percentage of the different burrow morphologies.Based on sex, male crabs were not only more prompted to make single Shaft and spiral burrows, but also constructed more complex burrows. Y-shaped and J-shaped burrows were more frequent in female crabs.
Figure 8 shows percentage of made burrows by sex during reproduction (5 males and 4 females) and non-reproduction period (8 males and 13 females).
Some differences were observed in frequency and morphology of burrows between foreshore and backshore. Most of the single Shaft burrows occurred in the foreshore zone during the reproduction period, with a mean opening diameter of 15.8 ± 6.0 mm, length of 265 ± 78.6 mm and α angle of 82.5°. J-shaped burrows had a mean opening diameter of 25 ± 14.5 mm, length of 537 ± 230.5 mm, depth of 404 ± 153 mm and α angle of 53.6°. For spiral burrows, opening diameter was 54.5 ± 7.91 mm, in length was 700 ± 149.9 mm, and depth reached to 660 ± 143.4 mm, with α angle being 57.3°. The last single Shaft burrow seaward distance was 480 cm away from shoreline in average 168.7 cm. This distance was 550 cm, 430 cm and 116 cm for last J-shaped, spiral and complex burrows seaward, respectively. The diameter, length and depth of single Shaft, J-shaped and Y-shaped burrows decreased with increasing distance from the shore during the reproductive period. Conversely, these parameters decreased increasing distance from the shore during non-reproductive period. This trend was similar to that observed in spiral burrows in the reproductive and non-reproductive period. Accordingly, diameter, length and depth of O. rotundata burrows increased landward. A similar result occurred for complex burrow (Table 3).
Most of the Y-shaped burrows were observed on the foreshore zone in the non-reproductive period, having a mean opening diameter of 15.8 ± 7.14 mm, length of 471 ± 277.8 mm, depth of 445 ± 241.3 mm, horizontal length of 176 ± 157.1 mm and α-angle of 61° and β-angle of 51.18°. The primary branch of all Y-shaped burrows was significantly longer than the secondary one, (t-statistics = 4.23, df = 16, P = 0.001), which joined together into a straight shaft with a mean length of 156 ± 53.3 mm. The secondary branch with a spherical end did not extend up to the surface. Primary branches faced seaward, whilst secondary faced landward. In most of the complex burrows, main entrance was divided into 2 shafts: one extended straight down and another turning right or left contrariwise to the first branch (Fig. 3J). These burrows had the largest mean diameter, with 65.8 ± 6.94 mm. Most of these burrows occurred on the foreshore zone during reproduction period, with a mean opening diameter 64.3 ± 7.06 mm. Ground angle for open branch was 50.66°.
The percentage of organic matter and moisture in relation to depth are shown in Tables 4 and 5, respectively. The results showed a decrease of moisture content from foreshore to backshore. The same result was observed for the percentage of organic matter in depths of 50–100 cm (Table 4). Maximum moisture content was obtained in depths of 50–100 cm for three studied zones (Table 5). Grain size showed variations in sediment composition, from fine to medium and coarse sand (Table 6). The mean value of pH was 8.1 where crabs were distributed.
Discussion
The trace fossil O. rotundata commonly occurred from the backshore to the foreshore of Salakh sediment with single shaft, J, Y, spiral and complex shaped. Increasing size of O. rotundata accompanied by morphological changes (e.g., shape, diameter, length, and orientation) of burrows. In this study, the burrows with smaller diameter mostly occurred on upper foreshore (Table 7), which can be due to inability of their gills to tolerate long time of air exposure. This pattern of burrow distribution was similar to that reported for O. cursor (Shuchman and Warburg 1978), O. ceratophthalmus (Chakrabarti 1981; Chan et al. 2006). The mean depth of single Shaft and J-shaped burrows on foreshore zone were 309 and 400 mm respectively which were more than the depths measured in O. quadrata on the coast of Texas (Hill and Hunter 1973), O. ceratophthalma in Hong Kong (Chan et al. 2006), India (Chakrabarti 1981) and Taiwan (Takahasi 1932). In our study, J-shaped burrows of juvenile crabs lacked any extra branch, as observed in India, Taiwan, America and Hong Kong (Takahasi 1932; Chakrabarti 1981; Chan et al. 2006). However, only two burrows had an extra branch, which is constructed by adult crabs. The orientation of the main branches was seaward, whilst the secondary branch was landward. This pattern was similar to crab populations in Hong Kong and India (Chakrabarti 1981; Chan et al. 2006). Conversely, none of the secondary branches of Y-shaped burrows opened to surface on Salakh beach, whilst a limited number of the secondary branches opened to surface on the Texas, India and Hong Kong coasts (Hill and Hunter 1973; Chakrabarti 1981; Chan et al. 2006). We hypothesize that O. rotundata constructed secondary branches in order to further safety and prevent predation which can be reason for low abundance of Y-shaped burrows in reproduction period. At the beginning of the reproduction period, from March to October (Naderi et al. 2018b), male crabs excavated complex burrows. Also, males created sand pyramids facing seaward in front of the burrows during daylight in high tide. Pyramids in front of the burrows of O. saratan and O. ceratophthalma were similar to those observed by Takahasi (1932) and Linsenmair (1967). Farrow (1971) stated that female crabs have Y-shaped burrows whilst males have spiral burrows; however, crabs of both sexes in O. rotundata were found to construct both Y-shaped and spiral burrows, as found in the study of Chan et al. (2006) on O. ceratophthalma. In our study, the trend of female crabs constructing mainly Y-shaped burrows whilst males made spiral burrows was different to the pattern noted by Chakrabarti (1981) in O. ceratophthalma.
With concerning to recruitment of O. rotundata from March to October (Naderi et al. 2018b), the main reason for high created single shaft burrow abundance is strong presence of megalopae (stage marks the passage from the sea to the beach (Smith 1873)) on foreshore zone where substrate moisture content is suitable for juvenile crabs.
During burrowing activities of O. rotundata, 4 types of sand disposal behaviour were observed: 1) Knocking sand with walking legs, 2) Throwing sand, 3) Stacking sand, which is similar to the observations of Schober and Christy (1993), Crane (1941), Vannini (1980) and Linsenmair (1967) and 4) Slamming sand with both chelae (especially major chelae) (Fig. 9) which done by male crabs. Like sand disposal behaviour of slamming, stacking sand was only made by male crabs, which is a sign for attraction of female crabs during the mating period (Schober and Christy 1993).
Salakh beach included coarse, medium and fine sand. The coarser granulometry between 0 and 50 cm depth on the shoreline zone could represent the accumulation of chopped oyster shells, coarse particles, etc. The results showed three grain size classes: symmetrical (foreshore and shoreline between 0–50 cm depth), coarse skewed (backshore between 0–50 cm depth) and very coarse skewed (shoreline and backshore between 50–100 cm depth). According to results, mating and reproductive behaviour of O. rotundata mainly occurred on the upper foreshore, with increasing diameter and length of burrows during the reproductive season. The reason for crabs migrating to the foreshore during reproductive period is related with the higher percentage of substrate moisture in comparison with backshore zone, which provide suitable substrate for burrowing and surpassing most of the egg development phase (Fellows 1973; Trott 1998; Negreiros-Fransozo et al. 2002). This could be an explanation for the relatively low number of ovigerous females found on the beach surface in several studies (Brooke 1981; Negreiros-Fransozo et al. 2002; Correa et al. 2014; Naderi et al. 2018b). Thus, the sand moisture could be a limiting factor in the distribution and abundance of ghost crabs, and hence an explanation of the low abundance of O. rotundata on the backshore zone throughout the year. On the other hand, ghost crabs are able to take up water from damp sand through the trichomes of setae, which are located between the third and fourth pereiopods (Wolcott 1978; Eshky 1985). In addition, none of the burrows investigated penetrate into the water table. As expected, the water content of sand decreased with distance from the sea, which was associated with an increase in burrow depth. Similar results were found on O. cursor (Hayasaka 1935; Warburg and Shuchman 1979; Williams 1983; Antia 1989; Strachan et al. 1999). Furthermore, Shinoda et al. (2019) found that crabs construct their burrows in appropriately wet zones.
The reason for curved burrows of O. rotundata depends on the heavy use of the same side for digging activity (Ali-Adnan 1985; Atkinson 1974). Conversely, Naderi et al. (2018b) obtained an equal number of left and right directions in burrows of O. rotundata. Other studies show similar results for O. ceratophthalma (Barrass 1963), O. saratan (Linsenmair 1967; Eshky 1985) and O. cursor (Strachan et al. 1999). We observed that the major claw was always the last part to access the burrows, which probably means that this part is used as a protective shield during resting time. In addition, Vannini (1980) observed this pattern for O. ryderi.
Burrows constructed (e.g., I, J, Y and spiral in shape) by O. rotundata in this study had a similar architecture to the results obtained in several studies on O. ceratophthalmus (Hayasaka 1935; Takahasi 1935; De 2005; Chan et al. 2006; Seike and Nara 2008; Lim et al. 2011), O. sinensis (Seike and Nara 2008), and O. rotundata (Zahedi et al. 2014), although some variability in shapes was evident. Lim et al. (2011) hypothesized that burrow shape was affected by sediment grain size. Also, they noted that Y-shaped burrows tend to be constructed in fine sediment, whereas J-shaped burrows are common in coarse granulometry. In our study, the casts of our burrows presented all kinds of shapes, occurring on the foreshore and backshore zone of Salakh beach, having coarse, medium and fine sediment. A similar pattern was observed on some beaches in Qeshm island (Kani, khorbas cave and Souza beaches) (Naderi unpublished data).
According to our results, O. rotundata individuals excavate single Shaft, J-shaped and Y-shaped burrows at the different growth stages, which was similar to observations reported by Lim et al. (2011). On the other hand, spiral and complex burrows only occurred in the adult crabs.
O. rotundata excavates burrows with different angles throughout the year cycle. The entrance branch of all burrow shapes was steeper during the reproduction period (Table 8), which can be a strategy of crabs for more safety and protection against predators. Also, the depth of all constructed burrows was bigger in non-reproduction period. In our study, we hypothesize that the increase in all depths of created burrows, in November and February, could be related to the reduction in weather. Lim et al. (2011) noted that steeper-sloped burrows in the opened branch of the Y-shaped burrows may provide better protection from predators since they provide a rapid retreat to deeper depths. This description is inconsistent as it fails to explain why J-shaped burrows do not present the same pattern. Results from our study showed that temporal variation had a significant impact on burrow slope. Also, Takahasi (1932) and Fellows (1966) pointed out that the tunnel in burrows becomes more horizontal with the increase in weight of the crab and substratum instability. In this study, we noted no relationship between those parameters and the angle of burrow entrance (Fig. 10).
According to semi-terrestrial O. rotundata, they closed their entrance of burrow as dome shaped before tidal inundations to provide a chamber of air at the top of the burrow. It seems that this activity could be helped to protect sand moisture in the burrow in order to renew their respiratory water (Fig. 11).
Generally, our results showed that temporal variation had significant impact on burrow slope, so that the entrance branch of all burrow shapes was clearly steeper during the reproduction period in contrast to the same burrows created in the non-reproduction period. Also, O. rutondata selects upper foreshore zone for mating and reproduction behavior.
Data Availability
All of data and materials are available and we can sent to Journal if are require.
References
Ali-Adnan E (1985) Aspects of the ecology, behaviour and physiology of the ghost crab Ocypode saratan (Forskål). Dissertation, University of Glasgow, Glasgow, United Kingdom
Antia EE (1989) Beach cusps and burrowing activity of crabs on a fine-grained sandy beach, southeastern Nigeria. J Coast Res 5(2):263–270
Atkinson RJA (1974) Behavioural ecology of the mud-burrowing crab Goneplax rhomboides. Mar Bio 25:239–252. https://doi.org/10.1007/BF00394970
Barrass R (1963) The burrows of Ocypode ceratophthalmus (Pallas) (Crustacea, Ocypodidae) on a tidal wave beach at Inhaca Island. Mozambique J Anim Ecol 32(1):73–85. https://doi.org/10.2307/2518
Blott SJ, Pye K (2001) GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf Process Landf 26(11):1237–1248. https://doi.org/10.1002/esp.261
Branco OJ, Hillesheim JC, Fracasso HAA, Christoffersen ML, Evangelista CL (2010) Bioecology of the Ghost Crab Ocypode quadrata (Fabricius, 1787) (Crustacea: Brachyura) Compared with Other Intertidal Crabs in the Southwestern Atlantic. J Shellfish Res 29(2):503–512. https://doi.org/10.2983/035.029.0229
Brooke ML (1981) Size as a factor influencing the ownership of copulation burrows by the ghost crab (Ocypode ceratophthalmus). Ethol Int J Behav Biol 55:63–78. https://doi.org/10.1111/j.1439-0310.1981.tb01259.x
Buchanan JB (1984) Sediment analysis. In: Holme NA, McIntyre AD (eds) Methods for the Study of Marine Benthos. Blackwell, Oxford, pp 41–65
Chakrabarti A (1981) Burrow pattern of Ocypode ceratophthalma (Pallas) and their environmental significance. J Paleontol 55(2):431–441
Chan BKK, Chan KKY, Leung PCM (2006) Burrow architecture of the ghost crab Ocypode ceratophthalma on a sandy shore in Hong Kong. Hydrobiologia 560:43–49. https://doi.org/10.1007/s10750-005-1088-2
Christoffers EW (1986) Ecology of the ghost crab Ocypode quadrata (Fabricius) on Assateague Island, Maryland and the impacts of various human uses of the beach on their distribution and abundance. Dissertation, Michigan State University, East Lansing, MI, USA
Correa MODA, Andrade LS, Costa CR, Castilho AL, Bertini G, Fransozo A (2014) Vertical distribution by demographic groups of ghost crab Ocypode quadrata (Crustacea: brachyuran). Biologia 67(7):905–915. https://doi.org/10.2478/s11756-014-0385-5
Crane J (1941) Eastern Pacific expeditions of the New York Zoological Society. XXIX. On the growth and ecology of brachyuran crabs of the genus Ocypode. Zoologica 26:297–310
Curran HA, White B (1991) Trace fossils of shallow subtidal to dunal ichnofacies in Bahamian Quaternary carbonates. Palaios 6(5):498–510. https://doi.org/10.2307/3514987
De C (2005) Biophysical model of intertidal beach crab burrowing: application and significance. An International Journal for Plant and Animal Traces 12(1):11–29. https://doi.org/10.1080/10420940590914471
Díaz H, Costlow JD (1972) Larval development of Ocypode quadrata (Brachyura: Crustacea) under laboratory conditions. Mar Biol 15:120–131. https://doi.org/10.1007/BF00353640
Eshky AA (1985) Aspects of the ecology, physiology and behavior of the crab Ocypode saratan on the Red Sea. Dissertation, University of Glasgow, Glasgow, United Kingdom
Farrow GE (1971) Back reef and lagoonal environment of Aldabra Atoll, distinguished by their crustacean burrows, In: Stoddart DR, Yonge CM (ed) Regional Variation in Indian Ocean Coral Reefs. London: Proceedings of the Symposium of the Zoological Society
Fellows DP (1966) Zonation and burrowing behaviour of the ghost crab Ocypode ceratophthalmus (Pallas) and Ocypode laevis Dana in Hawaii. Dissertation, University of Hawaii
Fellows DP (1973) Behavioral ecology of the ghost crab Ocypode ceratophthalmus and Ocypode cordimana at Fanning Atoll, Line Islands. In: Fanning Island Expedition, Chave KE, Kay EA (ed) Hawaii Institute of Geophysics Honolulu: University of Hawaii
Hayasaka I (1935) The burrowing activities of certain crabs and their geological significance. Am Midl Nat 16(1):99–103. https://doi.org/10.2307/2419880
Haley SR (1973) On the use of morphometric data as a guide to reproductive maturity in the ghost crab, Ocypode ceratophthalmus (Pallas) (Brachyura, Ocypodidae). Pac Sci 27:350–362
Haque H, Choudhury A (2014) Ecology and behavior of the ghost crab, Ocypode macrocera (Edwards 1834) occurring in the sandy beaches of Sagar Island. Sundarbans Int j Eng Sci Invention 3(4):38–43
Hartnoll R (1969) Matting in the Brachyura. Crustaceana 16:161–181. https://doi.org/10.1163/156854069X00420
Hill GW, Hunter RE (1973) Burrows of the ghost crab Ocypode quadrata (Facricius) on the Barrier Islands, southcentralTexas coast. J Sediment Petrol 43(1):24–30
Hughes DA (1973) On mating and the “copulation burrows” of crabs of the genus Ocypode (Decapoda, Brachyura). Crustaceana 24:72–79. https://doi.org/10.1163/156854073X00074
Jiang GC, Liu HC, Chan TY, Chan BKK (2014) First stage zoeal morphology of four ghost crabs Ocypode ceratophthalmus (Pallas, 1772), O. cordimanus Latreille, 1818, O. sinensis Dai, Song & Yang, 1985 and O. stimpsoni Ortmann, 1897 Crustacea, Decapoda, Ocypodidae. Zootaxa 3760(3):369–382. https://doi.org/10.11646/zootaxa.3760.3.4
Jones DA (1972) Aspects of the ecology and behaviour of Ocypode ceratophthalmus (Pallas) and Ocypode kuhlii de Haan (Crustacea: Ocypodidae). J Exp Mar Biol Ecol 8(1):31–43
Kristensen E, Penha-Lopes G, Delefosse M, Valdemarsen T, Quintana CO, Banta GT (2012) What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar Ecol Prog Ser 446:285–302. https://doi.org/10.3354/meps09506
Lim SSL, Yong AYP, Tantichodok P (2011) Comparison of burrow morphology of juvenile and young adult Ocypode ceratophthalmus from Sai Kaew. Thailand J Crust Biol 31(1):59–65. https://doi.org/10.1651/10-3314.1
Linsenmair KE (1967) Konstruktion und Signalfunktion der sand Pyramide der Reiterkrabbe Ocypode saratan Forsk (Decapoda Brachyura Ocypodidae). Ethology Int J Behav Biol 24:403–456. https://doi.org/10.1111/j.1439-0310.1967.tb01238.x
Lucrezi S, Schlacher TA (2014) The ecology of ghost crab. Oceanogr Mar Biol 52:201–256. https://doi.org/10.1201/b17143-5
Ma KY, Chow LH, Wong KJH, Chen HN, Ip BHY, Schubart CD, Tsang LM, Chan BKK, Chu KH (2019) Speciation pattern of the horned ghost crab Ocypode ceratophthalmus (Pallas, 1772): An evaluation of the drivers of Indo-Pacific marine biodiversity using a widely distributed species. J Biogeogr 45(4):2658–2668. https://doi.org/10.1111/jbi.13443
Mantelatto FLM, Fransozo A (1997) Fecundity of the crab Callinectes ornatus Ordway, 1863 (Decapoda, Brachyura, Portunidae) from the Ubatuba region, São Paulo, Brazil. Crustaceana 70:214–224.https://www.jstor.org/stable/20105853
McDermott JJ (2009) Notes on the unusual megalopae of the ghost crab Ocypode quadrata and related species (Decapoda: Brachyura: Ocypodidae). Northeast Nat 16(4):637–646. https://doi.org/10.1656/045.016.n413
Naderi M, Zare P, Lastra M, Pishehvarzad F (2018a) First record of ghost crab Ocypode sinensis (Dai Song and Yang, 1985) (Decapoda: Brachyura: Ocypodidae) from Qeshm Island, Persian Gulf, Iran. Cah Biol Mar 59:527–531. https://doi.org/10.21411/CBM.A.51F168A3
Naderi M, Hosseini SA, Hedayati AA, Pazooki J, Zare P, Lastra M (2018b) Reproductive biology of Ghost crab Ocypode rotundata (Miers, 1882) (Decapoda, Ocypodidae) of Qeshm Island (Persian Gulf). Crustaceana 91(9):1039–1059. https://doi.org/10.1163/15685403-00003804
Naderi M, Pishehvarzad F (2019) Morphological survey of the burrows of the ghost crab Ocypode rotundata (Miers, 1882) in the southwestern Qeshm Island. Iran Fish Sci J 28(4):1–5 (In Persian)
Naderi M, Hosseini SA, Hedayati AA, Pazooki J, Lastra M (2019) Study of some morphometric traits, condition factor and growth parameters of the ghost crab Ocypode rotundata Miers, 1882 in Qeshm Island, Persian Gulf. J Aquat Ecol 8:61–73 (In Persian)
Naderloo R, Turkay M (2012) Decapod crustaceans of the littoral and shallow sublittoral Iranian coast of the Persian Gulf: faunistics, biodiversity and zoogeography. Zootaxa 3374:1–67. https://doi.org/10.11646/zootaxa.3374.1.1
Naderloo R, Ebrahimnezhad S, Sari AR (2015) Annotated checklist of the decapod crustaceans of the Gulf of Oman, northwestern Indian Ocean. Zootaxa 4028:397–412. https://doi.org/10.11646/zootaxa.4028.3.5
Najafi A (2014) Population and reproductive biology of ghost crab Ocypode rotundata (Miers, 1882) in Chabahar, Oman Sea, Iran. Dissertation, University of Chabahar, Chabahar, Iran
Negreiros-Fransozo ML, Fransozo A, Bertini B (2002) Reproductive cycle and recruitment period of Ocypode quadrata (Decapode, ocypode) at a sandy beach in southeastern Brazil. J Crust Biol 22(1):157–161. https://doi.org/10.1651/0278-0372(2002)022[0157:RCARPO]2.0.CO;2
Robertson JR, Pfeiffer WJ (1982) Deposit-feeding by the ghost crab Ocypode quadrata (Fabricius). J Exp Mar Biol Ecol 56(2–3):165–177. https://doi.org/10.1016/0022-0981(81)90187-8
Sakai K, Turkay M (2013) Revision of the genus Ocypode with the description of a new genus, Hoplocypode (Crustacea: Decapoda: Brachyura). Mem Queensl Mus 56(2):665–793
Schlacher TA, Jager R, Nielsen T (2011) Vegetation and ghost crabs in coastal dunes as indicators of putative stressors from tourism. Ecol Indic 11(2):284–294. https://doi.org/10.1016/j.ecolind.2010.05.006
Schober UM, Christy JH (1993) Sand disposal of the painted ghost crab Ocypode gaudichaudii (Decapoda: Ocypodidae): a possible role in courtship. Ecol Indic 116:53–60. https://doi.org/10.1007/BF00350731
Seike K, Nara M (2008) Burrow morphologies of ghost crab Ocypode ceratophthalama and Ocypode sinensis in foreshore, backshore, and subenvironments of a sandy beach in Japan. Jour Geol Soc Japan 114(11):591–596. https://doi.org/10.5575/geosoc.114.591
Shinoda A, Fujiwara SI, Niiya H, Katsuragi H (2019) Physical constraints on sand crab burrows: Mechanical properties of wet sand explain the size and spatial distributions of burrows on beaches. PLoS ONE 14:1–20
Shuchman E, Warburg MR (1978) Dispersal, population structure and burrow shape of Ocypode cursor. Mar Biol 49:255–263. https://doi.org/10.1007/BF00391138
Smith SI (1873) The megalops stage of Ocypoda. Am J Sci 6:67–68
Strachan PH, Smith RC, Hamilton DAB, Taylor AC, Atkinson RJA (1999) Studies on the ecology and behavior of the ghost crab, Ocypode cursor in northern Cyprus. Bull Mar Sci 63(1):51–60. https://doi.org/10.3989/scimar.1999.63n151
Takahasi S (1932) On the burrows of Ocypode ceratophthalma Fabricius. Kwagaku 2:329–335 (in Japanese)
Takahasi S (1935) Ecological notes on the ocypodian crabs (Ocypodidae) in Formosa, Japan. Annot Zool Jpn 15:78–87
Trott TJ (1987) Chemoreception in the painted ghost crab Ocypode gaudichaudii H. Milne Edwards and Lucas (Brachyura: Ocypodidae): Implications for foraging. Zool Anz 218(5–6):295–303
Trott TJ (1998) On the sex ratio of the painted ghost crab Ocypode gaudichaudii H. Milne Edwards & Lucas, 1843 (Brachyura, Ocypodidae). Crustaceana 71:47–56. https://doi.org/10.1163/156854098X00761
Vannini M (1980) Researches on the coast of Somalia. The shore and dune of Sar Uanle. 27. Burrows and digging behavior in Ocypode and other crabs (Decapoda Brachyura). Monit Zool Ital 13(1):11–14. https://doi.org/10.1080/00269786.1980.11758547
Warburg MR, Shuchman E (1979) Experimental studies on burrowing Ocypode cursor (L.) (Crustacea: Ocypodidae), in response to sand moisture. Mar Behav Physiol 6(2):147–156. https://doi.org/10.1080/10236247909378561
Williams JA (1983) Environmental regulation of burrow depth distribution of the sand-beach Amphipod Talitrus saltator. Estuar Coast Shelf S 16(3):291–298. https://doi.org/10.1016/0272-7714(83)90146-4
Wolcott TG (1978) Ecological role of ghost crabs, Ocypode quadrata (Fabricius) on an ocean beach: scavengers or predators? J Exp Mar Biol Ecol 31:67–82. https://doi.org/10.1016/0022-0981(78)90137-5
Wong KJH, Shih HT, Chan BKK (2012) The ghost crab Ocypode mortoni George, 1982 (Crustacea: Decapoda: Ocypodidae): redescription, distribution at its type locality, and the phylogeny of East Asian Ocypode species. Zootaxa 3626:71–87. https://doi.org/10.11646/zootaxa.3550.1.5
Zahedi F (2014) Population dynamic of the ghost crab (Ocypode rotundata) in tidal zone of Jask port in Hormozgan. Dissertation, University of Hormozgan, Bandar Abbas, Iran
Acknowledgements
We would like to thank Hamid Reza Eshghi, Mashaallah Soroudi and Ali Salakhi for their assistance during field work.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Mojtaba Naderi and Fatemeh Pishehvarzad designed the study, performed the field work and wrote the manuscript. Parviz Zare and Mariano Lastra performed the statistical analyses, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to Participate
Informed consent was obtained from all the individuals participants included in the study.
Human and Animal Ethics
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naderi, M., Pishehvarzad, F., Zare, P. et al. Influence of Temporal Variation on Morphology and Architecture in Burrows of Ocypode Rotundata (Miers, 1882) on Salakh Beach of Qeshm Island, the Persian Gulf. Thalassas 39, 1131–1144 (2023). https://doi.org/10.1007/s41208-023-00582-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-023-00582-1