Abstract
Extensive exploration of lichen diversity in the tiny and remote tropical island of Réunion (Mascarene Archipelago, Indian Ocean) yielded two new species in the hyperdiverse lichen family Parmeliaceae. Morphological, anatomical and chemical characters and molecular inferences from 3 loci (ITS, nuLSU and mtSSU) strongly support their assignment to Hypotrachyna (subgenus Longilobae) and Remototrachyna. Hypotrachyna producta is further assigned to H. subgenus Longilobae and is newly reported for Réunion as well as Remototrachyna costaricensis.
Similar content being viewed by others
Introduction
The Parmeliaceae family represents one of the most well-known lichen families, being easily recognized by any informed naturalist or ecologist, with representatives used in biodiversity assessment, and environmental and climate monitoring world-wide (examples in Aragón et al. 2013; Hauck and Javkhlan 2009; Normann et al. 2010; Saipunkaew et al. 2007). It is a large, subcosmopolitan and very diverse family with 79 genera and over 2700 species currently recognized (Thell et al. 2012), of which the basal radiation occured between 60 and 74 MA ago (Amo de Paz et al. 2011). This group has been extensively studied worldwide by several researchers for more than five decades (Crespo et al. 2010). However, some regions, especially the remote tropics, require further study. This includes Réunion, a small (2512 km2) and remote island in the Mascarene Archipelago in the Indian Ocean. So far, 54 species representing 12 genera of the Parmeliaceae were recorded from the island by van den Boom et al. (2011).
Remototrachyna, is a recent segregate of this family based on molecular and morphological data. Species are distinguished in having a pored epicortex, broad, sub-irregular lobes with rounded apices, short, mostly dichotomously branched rhizines, scleroplectenchymatous cupulate exciple, and large ellipsoid ascospores (Divakar et al. 2010). It shows the centre of diversity on the Indian subcontinent and in South East Asia. So far, the genus was unknown from Réunion. More recently, the genus Hypotrachyna was reclassified and five subgenera are recognized viz. Cetrariastrum, Everniastrum, Longilobae, Parmelinopsis, and Sinuosae (Divakar et al. 2013). Species are characterized by a pored epicortex, narrow, sublinear to linear elongate lobes, with truncate apices, dichotomously branched rhizines, oval-ellipsoid ascospores and bifusiform conidia. So far, 19 species are reported from Réunion under the genus names Hypotrachyna and Parmelinopsis (Masson 2012; van den Boom et al. 2011), but a revision of species of the Hypotrachyna clade occuring on that island is in progress by the first author.
Traditionally, morphological and chemical features have been used for species delimitation in lichens in general and Parmeliaceae in particular. In some cases, it has been a matter of debate as morphotypes (distinguished by the means of reproduction, either with apothecia or not, and by means of vegetative dispersion, with soralia or isidia) and chemotypes (distinguished by the secondary metabolites produced in the medulla) were widely recognized as appropriate traits, diagnostic at the species level (Culberson and Hale 1973; Elix et al. 1986; Poelt 1972). Nonetheless, in the last decade, important additional molecular data have emerged for accurate species assessment and taxonomic re-evaluation, and are frequently used for species delimitation in Parmeliaceae, especially in the parmelioid groups (see the review by Crespo and Pérez-Ortega 2009 and Lumbsch and Leavitt 2011; see also Leavitt et al. 2011, 2012, 2013). Along with detailed morphological and chemical analysis, we use here a three-loci dataset (ITS, nuclear LSU and mitochondrial SSU) to clarify the taxonomic status of four species in the parmelioid genera Hypotrachyna and Remototrachyna from Réunion, two presumably undescribed (H. penduliloba, R. pandani) and two new to the lichen mycota of the island (H. producta, R. costaricensis).
Materials and methods
Material was collected during several field trips to Réunion in 2003, 2005, 2009, 2012 and 2013. Morphological and chemical characters were assessed following the protocol described by Masson (2012). In particular, ascopore measurements were made in tap water in the dead hydrated state (Baral 1992) and statistics are given as the arithmetical mean value (in italics and underlined) plus/minus (±) 1.96 × the standard deviation (SD; rounded up to the nearest 0.5 μm); values in parentheses represent observed minimum and maximum values and Q represents the length/width ratio. Statistics for the other anatomical measurements are given as the arithmetical mean value (in italics) between the observed minimum and maximum values (in parentheses). Definition and terminology of the apothecial layers follow Ferencova (2012). Secondary metabolites were studied by thin layer chromatography (TLC) under the standard procedure with the solvent systems A, B, C, E and G (Orange et al. 2010). The codes used for colours follow Online Auction Color Chart (Online Auction Color Chart Company 2004). Bioclimates of the localities were determined according to Rivas-Martínez and Rivas-Sáenz (2009). Material preserved in the private herbarium of the first author is referred to as “h”.
Accessions from GenBank have been retrieved to assess the identity and phylogenetic relationships of our material. We here provide detailed descriptions of all species dealt with in this paper, including previously described ones; we indeed suspect that many cryptic species are still to be detected within Parmeliaceae and, thus, detailed descriptions should be most useful for further studies.
Well-preserved lichen specimens lacking any visible symptoms of fungal infection were sampled for DNA isolation. Extraction of DNA and polymerase chain reaction (PCR) amplification were performed following the protocol of Cubero et al. (1999). The following primers were used: (a) for ITS: ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990); (b) for mtSSU: mrSSU1 and mrSSU3R (Zoller et al. 1999); (c) for nuLSU, as suggested at http://www.lutzonilab.net/primers: LIC2044, LR0R, LR3R, LR3, and LR6. Amplicons were sequenced by Macrogen® or by the GIGA sequencing platform of the University of Liège. Sequence fragments were assembled with Sequencher version 4.9 (Gene Codes Corporation, Ann Arbor, Michigan). Sequences were subjected to MEGABLAST searches to detect potential contamination. The sequences were aligned manually using MacClade 4.05 (Maddison and Maddison 2002). Ambiguous regions were delimited using the online version of GBlocks v 0.91b (Castresana 2000) at http://molevol.cmima.csic.es/castresana/Gblocks.html, allowing for gap positions within the final blocks, and carefully checked manually.
We assembled two matrices. Matrix 1 was assembled to detect the phylogenetic affinities within Hypotrachyna as re-circumscribed by Divakar et al. (2013), and the second one for the same purpose with the genus Remotrachyna (Divakar et al. 2010). Both matrices included sequences of three loci, nuLSU, ITS and mtSSU for representative species of both genera, including the material under study and collected in Réunion (Table 1).
Congruence between the three loci partitions in both matrices was assessed, with datasets considered congruent if relationships characterized by bootstrap proportions for maximum likelihood (ML) or posterior probabilities above 70 % or 0.95, respectively, were identical or at least not in direct conflict among the inferences from individual loci. Since all partitions were shown to be congruent, they were concatenated. The two matrices are deposited in TreeBASE under the accession numbers 16641 and 16642, respectively, for the Hypotrachyna matrix and the Remototrachyna one.
For each matrix, phylogenetic relationships were reconstructed based on ML and Bayesian inferences. We used RAxML 7.0.4 (Stamatakis 2006; Stamatakis et al. 2008) for the ML analysis on the CIPRES gateway (Miller et al. 2010). We used the GTRGAMMA model for searching of the final most likely tree and for bootstrapping; the model includes a parameter (Γ) for rate heterogeneity among sites, and no parameter for estimating the proportion of invariable sites was included. Support for each branch was evaluated using the “fast bootstrap” with 1000 pseudoreplicates. Bayesian analyses were carried out using the Metropolis-coupled Markov chain Monte Carlo method (MC3) in MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003). No prior values were assumed. Model selection was based on Divakar et al. (2010, 2013). Four parallel runs were performed, each using four independent chains (three heated and one cold chain), with a single tree saved every 1000th generation for a total of 6,000000 generations. The incremental heating scheme was set by default. We used TRACER v1.6 (Rambaut et al. 2013) to plot the log-likelihood values of the sample points against generation time, and to determine when stationarity was achieved. Consequently the first 600,000 generations were deleted as the burn-in of the chain. A majority-rule consensus tree with average branch lengths was constructed for the remaining trees using the sumt option of MrBayes. Phylogenetic trees were visualized using FigTree v1.2.3 (Rambaut 2009). Branches support values were considered significant when ML bootstrap (MLBS) was > 70 % and Bayesian posterior probabilities (PP) were > 95 %.
Results and discussion
Phylogenetic analyses
Six sequences of nuLSU, ITS and mtSSU were newly generated for this study and the GenBank accession numbers are provided in Table 1. Matrix 1 was composed of nuLSU, ITS and mtSSU sequences for 38 representative species of the genus Hypotrachyna, with Parmeliopsis hyperopta as the outgroup, following Divakar et al. (2013). A total of 1910 characters were included, 290 being potentially parsimony-informative. The single most likely tree had a likelihood score of –8381.828239. Matrix 2 was composed of nuLSU, ITS and mtSSU sequences for 21 examples of the genus Remototrachyna, with Bulbothrix decurtata and B. goebelii as the outgroup (Divakar et al. 2010): these two species are representative of the two different clades forming the paraphyletic genus Bulbothrix, resolved as being a sister to Remototrachyna. A total of 1696 characters were included, 217 being potentially parsimony-informative. The single most likely tree had a likelihood score of –5490.597347.
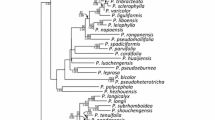
The phylogenetic affinities within Hypotrachyna sensu Divakar et al. (2013) could be confirmed, except for the subgenus Cetrariastrum that is poorly supported in both optimization analyses; the subgenus Longilobae is well resolved and a sister to all other subgenera, and the position of H. producta and our new species, here described as H. penduliloba, within the subgenus is strongly supported (Fig. 1). Both species share the same chemistry and can be distinguished by their morphology; furthermore, they differ in 11 bp substitutions in ITS1, 1 indel in 5.8S, and 4 indels plus 9 bp substitutions in ITS2 (data obtained from two thalli, one for each species). Except for the strongly supported clade formed by H. longiloba and H. densirhizinata, all other nodes within the subgenus are not supported.
Phylogenetic tree of the lichen genus Hypotrachyna including H. penduliloba sp. nov. and H. producta, both from Réunion material. Arrow points to subgenus Longilobae. 50 % consensus tree from MrBayes optimization, inferred from a three-loci matrix (ITS, nuLSU, mtSSU). Thickened branches with ML support > 70 % and Bayesian posterior probabilities > 0.95
The genus Remototrachyna was retrieved as strongly supported, with R. costaricensis, including our sample from Réunion, as a strongly supported clade sister to all other accessions (Divakar et al. 2010). Our new species, here described as R. pandani, comes next, resolved as a sister to all other taxa (Fig. 2), this node being strongly supported in the Bayesian analysis (ML-bootstrap = 72 %; PP = 1.0). The barcode ITS of three different collections displays some variation as there are five variable positions in ITS1, two in 5.8S, and one in ITS2. A much larger variation was detected between a GenBank accession of R. costaricensis (collected in Costa Rica) and our single collection from Réunion as they differ by 14 bp substitutions plus 1 indel in ITS1, and 17 plus 1 indel in ITS2.
Phylogenetic tree of the lichen genus Remototrachyna including H. pandani sp. nov. from Réunion and material of R. costaricensis from the same island. 50 % consensus tree from MrBayes optimization, inferred from a three-loci matrix (ITS, nuLSU, mtSSU). Thickened branches with ML support > 70 % and Bayesian Posterior Probabilities > 0.95; thickened branch in grey with support only in the Bayesian analysis (PP > 0.95)
Taxonomy
Hypotrachyna penduliloba D.M. Masson & Sérus., sp. nov. (Fig. 3)
a–f Hypotrachyna penduliloba. a Habit in the field (Masson 974.4361). b–d Linear soralia (pointed out with arrows) in various stages of development. b Plane lobe tips with young terminal soralia (Masson 974.4417). c Sinuous and involute lobe tips with terminal soralia (Masson 974.4420). d Sinuous lobe tip with subterminal soralium slightly spreading on the upper surface (Masson 974.4058). e Isotype in its natural habitat, a small patch of E. reunionensis-montane thicket in a cloud forest with abundant epiphytic moss mats. f Known distribution plotted on a topographic map of Réunion Island. Scale bars: a 2 cm, b–c 2 mm, d 1 mm, f 20 km
Mycobank MB 810751
Diagnosis
Hypotrachyna species belonging to subgenus Longilobae; thallus large (9–15 × 6–10 cm), loosely adnate and partially free hanging; lobes sublinear to linear, 0.4–3 mm wide, internodes 1–7 mm long; upper surface more or less white-maculate; soralia linear and terminal at first, then occasionally slightly spreading on the upper surface and subterminal; soredia subgranular, (30)–42.9–(70) μm in diameter; medulla thin (28–78 μm thick), white with anziaic acid; upper cortex with atranorin and chloroatranorin.
Type
France, Réunion, Le Tampon, le Volcan, trail to Piton Textor, 21°10′47″S, 55°38′13″E, 1870 m, on mossy branches of E. reunionensis, small patch of E. reunionensis-montane thicket in windward montane rainforest, 27 Aug. 2012, D. Masson 974.4087 (LG, holotype; G, isotype; GenBank Acc. for ITS: KP098555).
Description
Thallus foliose, epiphytic, (7)9–15 × (4)6–10 cm, loosely adnate and partially free hanging, membranaceous and fragile (Fig. 3a). Lobes sublinear to linear, separate to loosely imbricate, dichotomously to subdichotomously branched, 0.4–3 mm wide, internodes 1–7 mm long, sinuous or V-shaped axils, planar or sometimes slightly concave or convex, with entire and eciliate margins, subtruncate and occasionally slightly revolute apices. Upper surface whitish grey (oac123), almost white in central part, often brownish at lobe tip, more yellowish in the herbarium, with a narrow black marginal rim due to extension of the lower cortex along the edge, epruinose, without pseudocyphellae, more or less white-maculate, smooth and shiny near the apex, shallowly rugulose and rather dull towards the centre, sorediate, lacking pustules, dactyls and isidia. Lobules normally absent but marginal adventitious ones sometimes present in decaying parts. Soralia linear and terminal at first, the sorediate lobe tip becoming somewhat involute with the edge sinuate (Fig. 3b–c), then occasionally slightly spreading on the upper surface (Fig. 3d) and subterminal, the sorediate lobe tip becoming somewhat revolute. Soredia subgranular, (30)–42.9–(70) μm in diameter (n = 150, from 5 specimens, SD = 7.4 μm). Medulla white throughout. Lower surface black, more or less shiny, smooth to slightly rugulose, generally with a marginal zone (ca. 0.5–1.5 mm wide) chestnut brown at the non-sorediate lobe apices, whitish or buff variegated at the sorediate ones. Rhizines usually black, sometimes dark brown near the lobe tip, shiny, sparse to moderately dense, projecting beyond the lobe margins and forming a marginal fringe, ca. 0.4–1(1.5) mm long, dendroid, (2)3–5 times dichotomously or trichotomously branched. Apothecia and pycnidia not seen. Upper cortex palisade plectenchymatous, not fragile, (15)–20.1–(30) μm thick. Algal layer continuous or discontinuous, (13)–18.8–(25) μm thick. Medulla thin, (28)–44.7–(78) μm thick. Lower cortex paraplectenchymatous, (13)–15.7–(25) μm thick.
Chemistry
Spot tests and fluorescence: upper cortex K+ yellow, C-, KC-, P+ faintly yellow, UV-; medulla K-, C+ red, KC+ red, P-, UV-. Secondary metabolites (TLC): upper cortex with atranorin and chloroatranorin; medulla with anziaic acid.
Etymology
The specific name relates to the rather elongated lobes that often hang down.
Distribution and ecology
So far known from six localities in the central part of Réunion, on the southern side of the Piton des Neiges massif and the northwestern side of the Piton de la Fournaise massif (Fig. 3f). The localities lie between 1600 and 1870 m above sea level (a.s.l.) where the bioclimatic features can be summarized as follows: bioclimate: pluvial tropical; thermotype belt: upper mesotropical (332 ≤ It ≤ 393); ombrotype belts: from upper humid to ultrahyperhumid (11.6 ≤ Io ≤ 31.6). All thalli of H. penduliloba were found among mosses or on moss mats on bark of the endemic tree heather Erica reunionensis E.G.H. Oliv. [= Philippia montana (Willd.) Klotzsch] in E. reunionensis-montane thicket or montane rainforest (Fig. 3e). The filmy fern Hymenophyllum inaequale (Poir.) Desv. frequently grows intermixed with H. penduliloba thalli. Hypotrachyna penduliloba can be described as an aero- and substrato-hygrophilous, ombrophilous, moderately photophilous, acidophilous lichen.
Comments
Hypotrachyna penduliloba belongs to the subgenus Longilobae within Hypotrachyna species but its phylogenetic relation within this subgenus remains unresolved (Fig. 1). Subgenus Longilobae includes all Hypotrachyna species with anziac acid as the major medullary secondary metabolite as well as atranorin and chloroatranorin in the upper cortex, namely: H. halei, H. partita, H. producta and H. prolongata (Divakar et al. 2013; present work). Besides the similar chemistry, they share sublinear lobes and a scarcity of sexual reproduction; apothecia are unknown in most species, except for H. prolongata in which they occur occasionally and produce only immature spores (Sipman et al. 2009). Hypotrachyna partita Hale and H. prolongata (Kurok.) Hale are both isidiate-lobulate and are not known outside the Americas. The neotropical H. halei Sipman, Elix & T.H. Nash lacks vegetative propagules (Flakus et al. 2012; Sipman et al. 2009). Hypotrachyna producta Hale is more widespread, being also known from outside America (see below). It is a sorediate species like H. penduliloba but the soralia are subapical-subcapitate and their ontogeny are quite different from those of H. penduliloba. In H. producta, the lobe tip first becomes revolute and, very often, even cucullate, and eventually the soralium develops progressively on the convex dorsal side (Fig. 4b–c). This development pattern is very frequent amongst the sorediate Hypotrachyna species. In H. penduliloba, in contrast, soredia appear at the lobe tip margin and form a linear soralium restricted to the lobe tip (Fig. 3b) that can become somewhat involute with a sinuate margin (Fig. 3c). Ultimately the soralium may slightly spread over the upper surface (Fig. 3d), with the sorediate lobe tip becoming somewhat revolute. To our knowledge, this kind of soredia development has not been described in the genus Hypotrachyna.
The lobes of H. penduliloba are often distinctly elongated and pendulous. This growth form is unusual among the Hypotrachyna species of Réunion even if some specimens of H. laevigata (Sm.) Hale may develop a mixture of linear and sublinear lobes. Linear, free-hanging lobes with relatively long internodes rarely occur in East Africa (Krog and Swinscow 1979) or in Papua New Guinea (Louwhoff and Elix 2002) but are more frequent among the representatives of that genus in the mountains of Central and South America (Hale 1975; Sipman et al. 2009). According to Sipman (2002), this lobe configuration might be an adaptation to high humidity, allowing a more rapid drying of the thallus and, thus, maintaining the equilibrium between alga and fungus in humid conditions. Interestingly, H. penduliloba thalli from the Piton de la Fournaise localities, with approximately 4–5 m average annual rainfall (Jumaux et al. 2011), have clearly more elongated lobes than those from the Piton des Neiges localities, with ca. 2–4 m average annual rainfall.
Additional specimens examined
France, Réunion, Cirque de Cilaos, track to Taïbit pass, 21°06′50″S, 55°26′28″E, 1600–1700 m, on trunk of Erica, thickets with Hypericum lanceolatum and Sophora denudata, 13 Nov. 2009, N. Magain & E. Sérusiaux s.n. (LG); La Plaine-des-Palmistes, trail to Piton des Cabris, 21°09′47″S, 55°39′17″E, 1655 m, on mossy branch of E. reunionensis, E. reunionensis-montane thicket in a small valley, 21 Aug. 2013, D. Masson 974.4361 (h); Le Tampon, Plaine des Cafres, trail to Bébour, 21°08′34″S, 55°34′18″E, 1600 m, on mossy branchlet of E. reunionensis, somewhat human-disturbed E. reunionensismontane thicket, 26 Aug. 2012, D. Masson 974.4058 (h); Le Tampon, forêt de la Plaine des Cafres, GR R2 trail, 21°09′03″S, 55°32′42″ E, 1740 m, on mossy boles of old E. reunionensis, E. reunionensis-montane thicket, 23 Aug. 2013, D. Masson 974.4416, 974.4417, 974.4418 (h, REU); Le Tampon, forêt de la Plaine des Cafres, GR R2 trail, 21°08′58″S, 55°32′38″ E, 1755 m, on mossy branch of E. reunionensis, E. reunionensis-montane thicket, 23 Aug. 2013, D. Masson 974.4420 (h).
Hypotrachyna producta Hale, Smithson. Contr. Bot. 25: 56 (1975) (Fig. 4)
Description
Thallus foliose, corticolous, 10 × 8 cm, adnate to moderately adnate (Fig. 4a). Lobes sublinear, contiguous to imbricate, dichotomously to subdichotomously branched, 1–5 mm wide, internodes 1–3 mm long, sinuous axils, planar or concave, with entire and eciliate margins, subtruncate to more or less rounded apices. Upper surface whitish grey (oac81 or oac123), sometimes brownish at the extreme lobe tip, more yellowish in the herbarium, narrow black marginal rim due to extension of the lower cortex along the edge, epruinose, without pseudocyphellae, in places faintly white-maculate, shallowly rugulose throughout, shiny near the apex and rather dull towards the centre, sorediate, lacking pustules, dactyls, lobules and isidia. Soralia subcapitate, subapical, forming dorsally at the cucullate tip of short lateral lobes (Fig. 4b–c). Soredia subgranular, (30)–43.5–(60) μm in diameter (n = 50, from one specimen, SD = 6.2 μm). Medulla white throughout. Lower surface black, more or less shiny, smooth to slightly rugulose, generally with a marginal chestnut brown zone (ca. 0.5–2 mm wide) at the lobe apices. Rhizines are usually black, sometimes dark brown near the lobe tip, shiny, fragile, sparse to dense, in places projecting beyond the lobe margins and forming a marginal fringe, ca. 0.3–1 mm long, dendroid, 2–5 times dichotomously to irregularly branched. Apothecia and pycnidia absent. Upper cortex palisade plectenchymatous, (15)–17.2–(20) μm thick. Algal layer more or less continuous, (20)–22.8–(25) μm thick. Medulla (40)–52.8–(73) μm thick. Lower cortex paraplectenchymatous, (13)–14.4–(18) μm thick.
Chemistry
Spot tests and fluorescence: upper cortex K+ yellow, C-, KC-, P- or P+ faintly yellow, UV-; medulla K-, C+ red, KC+ red, P-, UV-. Secondary metabolites (TLC): upper cortex with atranorin and chloroatranorin; medulla with anziaic acid.
Distribution and ecology
Known from Réunion from a single thallus collected at an elevation of 1735 m in a montane cloud forest [leeward mountain rainforest according to Strasberg et al. (2005)] covering a southeast facing slope above Îlet des Salazes in the Cilaos cirque. The bioclimatic features of the locality are: bioclimate: pluvial tropical; thermotype belt: upper mesotropical (It = 372); ombrotype belt: upper humid (Io = 10.8). The thallus thrived on bark of an old tree heather Erica reunionensis.
Specimens examined
Colombia, Huila, La Plata, Vereda La Candelaria, headwaters of Rio La Candelaria, 2300 m, Blechnum-Sphagnum bog with scattered shrubs, 01 Oct. 1984, J. Aguirre C. & H.J.M. Sipman 6151, 6165 (B). Costa Rica, San José, E of Cerro Buenavista, Cerro de la Muerte, along the Panamerican Highway, 9°33′N, 83°46′W, 3400 m, epiphyte, ca. 2-m tall scrub with rock outcrops, 23 March 1985, H.J.M. Sipman 20.938 (B). France, Réunion, Cilaos, above Îlet des Salazes, 21°06′33″S, 55°26′46″E, 1735 m, corticolous on branch of old E. reunionensis, leeward montane rainforest, 20 Aug. 2012, Masson 974.3907 (h).
Comments
Hypotrachyna producta is characterized by sublinear lobes with subapical, subcapitate soralia on short lateral lobes, atranorin and chloroatranorin in the cortex and anziaic acid in the medulla (Hale 1975; Krog and Swinscow 1979; Sipman et al. 2009). Known from southeastern USA and the Neotropics, New Zealand (Sipman et al. 2009), and Kenya and Uganda in Africa (Krog and Swinscow 1979), H. producta is a new addition to the lichen flora of Réunion as well as the Mascarene Archipelago. The phylogenetic position of H. producta remains unresolved within the subgenus Longilobae in our molecular study (Fig. 1).
Remototrachyna costaricensis (Nyl.) Divakar & A. Crespo, Am. J. Bot. 97: 586 (2010) (Fig. 5)
Description
Thallus foliose, corticolous, 11 × 9 cm, moderately adnate. Lobes irregular, contiguous to imbricate, 1.5–5 mm wide, convex or concave, rarely planar, with entire and eciliate margins, subtruncate to more or less rounded apices, sinuous axils. Upper surface whitish grey (oac123), rarely brownish at the extreme lobe tip, more yellowish in the herbarium, narrow black marginal rim due to extension of the lower cortex along the edge, patchily distinctly white-pruinose in the central parts of the lobes, without pseudocyphellae, in places faintly white-maculate, rugulose throughout, more or less shiny near the apex, dull towards the centre, isidiate, lacking pustules, dactyls, lobules and soredia. Isidia laminal, rarely submarginal, unevenly distributed, subglobose at first, then irregularly cylindrical or slightly inflated, more or less branched and coralloid (to 0.6 mm high), epruinose, apices syncorticate, brown, eciliate. Medulla white throughout. Lower surface black, more or less shiny, rugulose, with a marginal chestnut brown zone (ca. 1–2 mm wide) at lobe apices. Rhizines black, paler near the tip, shiny, moderately dense to dense, in places projecting beyond the lobe margins and forming a marginal fringe, ca. 0.2–0.5 mm long, dendroid. Apothecia absent. Pycnidia frequent, laminal towards lobe tips, black, immersed. Conidia not seen. Upper cortex palisade plectenchymatous, (15)–20.2–(25) μm thick. Algal layer more or less continuous, (23)–24.2–(25) μm thick. Medulla (58)–64.2–(70) μm thick. Lower cortex paraplectenchymatous, (18)–19.4–(23) μm thick.
Chemistry
Spot tests and fluorescence: upper cortex K+ yellow, C-, KC-, P- or P+ faintly yellow, UV-; medulla K-, C-, KC-, P-, UV-. Secondary metabolites (TLC): upper cortex with atranorin and chloroatranorin; medulla with fatty acids of the constipatic acid complex.
Distribution and ecology
Known in Réunion from a single thallus collected at 1600 m elev. on a mossy branch of the endemic shrub Hubertia tomentosa Bory, near a gully, in a somewhat human-disturbed Erica reunionensis-montane thicket. Bioclimate of the locality is pluvial tropical in the upper mesotropical thermotype belt (It = 375) and the hyperhumid ombrotype belt (Io = 25.9).
Specimen examined
France, Réunion, Le Tampon, Plaine des Cafres, trail to Bébour, Bras Clair, 21°08′34″S, 55°34′18″ E, 1600 m, corticolous on branch of Hubertia tomentosa, Erica reunionensis-montane thicket near a gully, 26 Aug. 2012, D. Masson 974.4056 (h).
Comments
Both morphological and chemical characters of the specimen from Réunion match well with Remototrachyna costaricensis (detailed descriptions in Louwhoff and Elix 2002 and Sipman et al. 2009). Furthermore, in the present phylogenetic tree it clustered with another accession of R. costaricensis from Costa Rica. The monophyly of the species is strongly supported (Fig. 2). The development of pruina on the upper surface as well as maculation seem rather variable in this species: pruina was absent and maculation was more or less present in specimens from Papua New Guinea (Louwhoff and Elix 2002), slight pruina near the tips and usually strong maculation in tropical America (Sipman et al. 2009), occasional pruina and faint maculation in specimens from Ecuador (Yánez-Ayabaca 2009), locally dense pruina and faint maculation in specimens from Brazil (Canêz 2005), and pruina in the central parts of the lobes and faint maculation in the specimen from Réunion. Thalli with fatty acids of the constipatic acid complex in the medulla are known from Mexico to Brazil in America (Nash et al. 2002; Sipman et al. 2009), Kenya and Tanzania in East Africa (Krog and Swinscow 1979), Papua New Guinea (Louwhoff and Elix 2002) and Réunion. Specimens with protolichesterinic acid as the major medullary substance and/or with an undetermined fatty acid (possibly caperatic acid) are also mentioned [as Parmelia costaricensis Nyl. or Hypotrachyna costaricensis (Nyl.) Hale] from several localities such as India (Patwardhan and Prabhu 1977; Divakar and Upreti 2005), Malaysia (Sipman 1993), Australia (Elix 1994), New Zealand (Galloway 1985), East Africa (Krog and Swinscow 1979), Brazil (Eliasaro et al. 1998), Ecuador (Yánez-Ayabaca 2009), Azores (Arvidsson 1990), southeast USA (Harris 1993), etc. Further detailed studies are needed to confirm that they all belong to R. costaricensis. Indeed, cryptic speciation may be hidden under a single epithet as an impressive variation in the barcode ITS has been detected between an accession retrieved from GenBank (material collected in Costa Rica) and our collection from Réunion: 14 bp substitutions plus 1 indel in ITS1 and 17 plus 1 indel in ITS2. Although no detailed study has been conducted on the genetic distances within this genus, the threshold to distinguish species boundaries as highlighted by Del-Prado et al. (2010) for several genera in the Parmeliaceae is crossed.
Remototrachyna pandani D.M. Masson & Sérus., sp. nov. (Fig. 6)
a–e Remototrachyna pandani. a Habit (part of holotype). b Thallus with apothecia (Masson 974.4447). c cross-section through the apothecium in lactic cotton blue, HY = hymenium, SH = subhymenium, HL = hyaline layer, IL = intermediate layer, BL = basal layer, AL = algal layer (holotype). d Known distribution plotted on a topographic map of Réunion. e Pandanus submontane wet thicket with the endemic tree Pandanus montanus, the most frequent phorophyte (type locality). Scale bars: a 1 cm, b 5 mm, c 20 μm, d 20 km
Mycobank MB 810752
Diagnosis
Species belonging to the genus Remototrachyna according to apothecium anatomy and molecular inferences; thallus corticolous of medium size (6–9 × 3–5 cm), adnate to tightly adnate; lobes sublinear to subirregular, 1–3 mm wide; upper surface often white-pruinose; soralia subcapitate, subapical on short lateral lobes; soredia farinose, (20)–27.3–(40) μm in diameter; apothecia rare, up to 4 mm in diameter, margin crenate and sorediate; ascospores broadly ellipsoidal to ellipsoidal, (8)8.5–10.1–11.5(12) × 5–6.0–7(7.5) μm; medulla white, with protocetraric acid and often an undetermined pigmentosin; upper cortex with atranorin and chloroatranorin.
Type
France, Réunion, La Plaine-des-Palmistes, Ancienne Nationale, 21°06′36″S, 55°39′29″E, 775 m, on branches or trunks of Pandanus montanus, somewhat human-disturbed Pandanus submontane wet thicket, 24 Aug. 2013, D. Masson 974.4433 (LG holotype; G, UPS, herb. D. Masson, isotypes; GenBank Acc. for ITS: KP098544).
Description
Thallus foliose, corticolous, (4)6–9(13) × 3–5(8) cm, adnate to tightly adnate (Fig. 6a). Lobes separate to subimbricate, sublinear to subirregular, subdichotomously to irregularly branched, sinuous axils, (0.5)1–3(4) mm wide, more or less planar near tips, uneven in central parts, with eciliate, sinuous, occasionally somewhat involute margins, subrotund to subtruncate apices. Upper surface whitish grey to yellowish grey (oac30, oac67, oac81, oac123), more yellowish in the herbarium, at times dark olive-greenish at the lobe tip, narrow black marginal rim due to extension of the lower cortex along the edge, often (68 % of the thalli) with laminal, densely pruinose patches, mostly more or less scattered in young parts but sometimes extending to all parts and covering large areas, without pseudocyphellae, emaculate or faintly white-maculate, smooth and shiny near the apex, rather dull and usually transversely cracked towards the centre, sorediate, lacking pustules, dactyls and isidia. Lobules normally absent but marginal, short (up to 1 mm long), more or less spatulate ones sometimes present. Soralia subcapitate, subapical on short lateral lobes, very rarely laminal, shortly pedicellate and elevated at maturity, often coalescing, evenly distributed or mainly developing in the central parts of the thallus, occasionally with a yellowish tint. Soredia farinose, (20)–27.3–(40) μm in diameter (n = 150, from 5 specimens, SD = 5.5 μm). Medulla white throughout. Lower surface black, shiny, rugulose, with a marginal zone (ca. 0.5–1.5 mm wide) cinnamon brown at lobe apices. Rhizines black, more or less shiny, moderately dense, projecting beyond the lobe margins and forming a marginal fringe, ca. 0.2–0.7(1) mm long, dendroid, (1)2–5 times dichotomously to irregularly branched. Apothecia rare (fertile thalli found in two localities out of nine), laminal, sessile or rarely substipitate, up to 4 mm in diameter, disc purple brown (oac601), more or less glossy, first concave or plane, rarely convex, finally undulate contorted and somewhat radially split, margin crenate and soon sorediate (Fig. 6b), epihymenium (5)–6.6–(10) μm high, hymemium (33)–37.5–(45) μm high, subhymenium (15)–20.0–(25) μm high, proper exciple of type I (Ferencova 2012), hyaline layer (10)–14.4–(18) μm high, intermediate layer (13)–16.9–(20) μm high, cortex-like basal layer with hyphae predominantly vertically arranged, interwoven, made up of cells with thick walls and elongated and flexuous lumen, (23)–33.1–(43) μm high (Fig. 6c). Ascospores 8 per ascus, simple, colourless, broadly ellipsoidal to ellipsoidal, (8)8.5–10.1–11.5(12) × 5–6.0–7(7.5) μm, Q = (1.33)1.35–1.70–2.05 (n = 60), epispore ca. 1 μm thick. Pycnidia not seen. Upper cortex palisade plectenchymatous, (10)–16.5–(28) μm thick. Algal layer continuous, (13)–18.6–(23) μm thick. Medulla (40)–53.5–(65) μm thick. Lower cortex paraplectenchymatous, thin, (8)–11.5–(15) μm thick.
Chemistry
Spot tests and fluorescence: upper cortex K+ yellow, C-, KC-, UV-; medulla K-, C-, KC± pink, P+ orange, UV-. Secondary metabolites (TLC): upper cortex with atranorin and chloroatranorin; medulla with protocetraric acid and often an undetermined pigmentosin (Rf A: 50–54, B: 28–30, C: 59–64, G: 75)
Etymology
Named after the most frequent phorophyte, the endemic tree Pandanus montanus Bory.
Distribution and ecology
So far known from nine localities in the windward part of Réunion from both the Piton des Neiges massif and the Piton de la Fournaise massif (Fig. 6d). The bulk of localities lies between 685 and 945 m elev. where the bioclimatic features can be summarized as follows: bioclimate: pluvial tropical; thermotype belts: upper thermotropical and lower mesotropical (478 ≤ It ≤ 540); ombrotype belts: from lower hyperhumid to ultrahyperhumid (18.0 ≤ Io ≤ 50.0). One locality differs by upper elevation (1385 m) and upper mesotropical thermotype belt (It = 376). The main habitat type is the Pandanus submontane wet thicket (Fig. 6e) but Remototrachyna pandani can also thrive in the windward submontane or montane rainforests. The endemic screw-pine Pandanus montanus Bory is the principal phorophyte (86 % of the observations); R. pandani was also found on several other trees species such as Monimia rotundifolia Thouars. It grows on bark of branches or trunks, or even on adventitious roots of P. montanus. Remototrachyna pandani seems to be an aero-hygrophilous, ombrophilous, fairly photophilous, moderately thermophilous lichen.
Comments
Amongst the 19 species of Remototrachyna currently known (Divakar et al. 2010; Flakus et al. 2012), R. pandani is the only sorediate one with protocetraric acid as the main medullary extrolite. Five other Remototrachyna species contain protocetraric acid in the medulla but three of them, R. consimilis (Vain.) Flakus, Kukwa & Sipman, R. koyaensis (Asahina) Divakar & A. Crespo and R. sipmaniana Kukwa & Flakus, are isidiate and the other two, R. adducta (Nyl.) Divakar & A. Crespo and R. aguirrei (Sipman, Elix & T.H. Nash) Flakus, Kukwa & Sipman lack vegetative propagules. Further, R. adducta, R. aguirrei and R. koyaensis have longer (>12 μm) and larger (>7 μm) ascospores; R. aguirrei, R. koyaensis and R. sipmaniana have wider lobes: 2–7 mm (Sipman et al. 2009), 2–8 mm (Divakar and Upreti 2005; Louwhoff and Elix 2002) or 4–10 mm (Hale 1975), and 5–8(10) mm (Flakus et al. 2012) wide, respectively. In overall morphology and chemistry, R. pandani closely resembles Hypotrachyna pseudosinuosa (Asahina) Hale and the two are likely to be easily confused. According to Divakar et al. (2010) the major morphological-anatomical character separating Remototrachyna from Hypotrachyna is the ascoma anatomy, especially the structure of the proper exciple (see also Ferencova 2012; Flakus et al. 2012). Unfortunately H. pseudosinuosa seems to be very rarely fertile and the detailed anatomy of its apothecia is still unknown. H. pseudosinuosa has been described from Japan (Asahina 1951) and has been mentioned from various parts of the world ever since (see Masson 2005 for a review). However, the morphological variability observed throughout its range may reflect cryptic speciation that requires further research (Louwhoff and Elix 2002; our observations). The loci ITS, nuLSU and mtSSU of two H. pseudosinuosa specimens from China were examined by Divakar et al. (2010, 2013) and it appears that they belong to the Hypotrachyna sensu stricto clade; they are thus clearly distinct from the new species R. pandani.
Additional specimens examined
France, Réunion, Bras-Panon, Plaine des Lianes, 21°01′50″S, 55°35′39″E, 880 m, on bark of Pandanus montanus, Pandanus submontane wet thicket, 25 Jul. 2005, D. Masson 974.1744 (h); Bras-Panon, Plaine des Lianes, 21°02′05″S, 55°35′45″E, 865 m, on branch of Pandanus montanus, Pandanus submontane wet thicket, 29 Aug. 2012, D. Masson 974.4121 (h); La Plaine-des-Palmistes, l’Ancienne Nationale, 21°06′35″S, 55°39′32″E, 770 m, on branches of Pandanus montanus, somewhat human-disturbed Pandanus submontane wet thicket, 24 Aug. 2013, D. Masson 974.4431, 974.4432 (h, REU); La Plaine-des-Palmistes, Ligne Deux Mille en Dessous, 21°07′00″S, 55°39′05″E, 870 m, on branches of Pandanus montanus, somewhat human-disturbed Pandanus submontane wet thicket, 24 Aug. 2013, D. Masson 974.4446, 974.4447 (h); Saint-André, forêt communale, 20°59′39″S, 55°35′33″E, 770 m, on trunk of an undetermined tree, windward submontane rainforest, 28 Jul. 2005, D. Masson 974.1807 (h); Saint-Benoît, Saint-François les Hauts, Sainte-Marguerite trail, 21°06′57″S, 55°40′42″E, 685 m, on branch and trunk of Pandanus montanus, Pandanus submontane wet thicket, 28 Aug. 2012, D. Masson 974.4099, 974.4107 (h); Saint-Benoît, Piton de Bébour, 21°07′33″S, 55°33′53″E, 1385 m, on branch of Monimia rotundifolia, windward montane rainforest, 07 Apr. 2003, D. Masson 974.0125 (h); Saint-Philippe, Saint-Philippe forest, trail to Piton Ravine Basse Vallée, 21°19′47″S, 55°42′12″E, 945 m, on root of Pandanus montanus, windward submontane rainforest, 16 Aug. 2013, D. Masson 974.4257 (h); Sainte-Rose, Mourouvin forest, Réservoirs trail, 21°09′37″S, 55°45′36″E, 880 m, on branch of Pandanus montanus, somewhat human-disturbed Pandanus submontane wet thicket, 15 Aug. 2013, D. Masson 974.4231 (h).
References
Amo de Paz G, Cubas P, Divakar PK, Lumbsch HT, Crespo A (2011) Origin and diversification of major clades in parmelioid lichens (Parmeliaceae, Ascomycota) during the Paleogene inferred by Bayesian analysis. PLoS ONE 6(12):e28161
Aragón G, Belinchón R, Martínez I, Prieto M (2013) Estimating epiphytic lichen richness by single families in Mediterranean forests. For Ecol Manag 310:187–193
Arvidsson L (1990) Additions to the lichen flora of the Azores. Bibl Lichenol 38:13–27
Asahina Y (1951) Lichenes Japoniae novae vel minus cognitae (7). J Jap Bot 26:329–334
Baral HO (1992) Vital versus herbarium taxonomy: morphological differences between living and dead cells of Ascomycetes, and their taxonomic implications. Mycotaxon 44:333–390
Canêz LS (2005) A família Parmeliaceae na localidade de Fazenda da Estrela, município de Vacaria, Rio Grande do Sul, Brasil. Mastership dissertation, Instituto de Botânica, São Paulo. Available at : http://www.biodiversidade.pgibt.ibot.sp.gov.br/Web/teses/2005/pdf/Luciana_da_Silva_Canez_MS.pdf
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Crespo A, Pérez-Ortega S (2009) Cryptic species and species pairs in lichens: a discussion on the relationship between molecular phylogenies and morphological characters. Anales Jard Bot Madrid 66(suppl 1):71–81
Crespo A, Kauff F, Divakar PK, Del-Prado R, Pérez-Ortega S, de Paz GA, Ferencova Z, Blanco O, Roca-Valiente B, Núñez-Zapata J, Cubas P, Argüello A, Elix JA, Esslinger TL, Hawksworth DL, Millanes AM, Molina MC, Wedin M, Ahti T, Aptroot A, Barreno E, Bungartz F, Calvelo S, Candan M, Cole MJ, Ertz D, Goffinet B, Lindblom L, Lücking R, Lutzoni F, Mattsson JE, Messuti MI, Miadlikowska J, Piercey-Normore MD, Rico VJ, Sipman H, Schmitt I, Spribille T, Thell A, Thor G, Upreti DK, Lumbsch HT (2010) Phylogenetic generic classification of parmelioid lichens (Parmeliaceae, Ascomycota) based on molecular, morphological and chemical evidence. Taxon 59:1735–1753
Cubero OF, Crespo A, Fatehi J, Bridge PD (1999) DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized, and other fungi. Plant Syst Evol 216:243–249
Culberson CF, Hale ME Jr (1973) Chemical and morphological evolution in Parmelia sect. Hypotrachyna: product of ancient hybridization ? Brittonia 25:162–173
Del-Prado R, Cubas P, Lumbsch HT, Divakar PK, Blanco O, Amo de Paz G, Molina MC, Crespo A (2010) Genetic distances within and among species in monophyletic lineages of Parmeliaceae (Ascomycota) as a tool for taxon delimitation. Mol Phylogenet Evol 56:125–133
Divakar PK, Upreti DK (2005) Parmelioid lichens in India (A revisionary study). Bishen Singh Mahendra Pal Singh, Dehra Dun
Divakar PK, Lumbsch HT, Ferencova Z, Del-Prado R, Crespo A (2010) Remototrachyna, a newly recognized tropical lineage of lichens in the Hypotrachyna clade (Parmeliaceae, Ascomycota), originated in the Indian subcontinent. Am J Bot 97:579–590
Divakar PK, Crespo A, Núñez-Zapata J, Flakus A, Sipman HJM, Elix JA, Lumbsch HT (2013) A molecular perspective on generic concepts in the Hypotrachyna clade (Parmeliaceae, Ascomycota). Phytotaxa 132:21–38
Eliasaro S, Adler MT, Elix JA (1998) The species of Hypotrachyna (Parmeliaceae, lichenized Ascomycotina) from the Segundo Planalto in the state of Parana, Brazil. Mycotaxon 69:255–270
Elix JA (1994) Hypotrachyna. Flora Aust 55:49–59
Elix JA, Johnston J, Armstrong PM (1986) A revision of the lichen genus Xanthoparmelia in Australasia. Bull Br Mus Nat Hist Bot 15:163–362
Ferencova Z (2012) Estudio morphológico comparado de los caracteres generativos en relación con los linages monofiléticos de la familia Parmeliaceae (Lecanorales, Ascomycota). Ph D thesis, Universidad Complutense de Madrid. Available at: http://eprints.ucm.es/22628/1/T34425.pdf
Flakus A, Saavedra PR, Kukwa M (2012) A new species and new combinations and records of Hypotrachyna and Remototrachyna from Bolivia. Mycotaxon 119:157–166
Galloway DJ (1985) Flora of New Zealand lichens. Government Printer, Wellington
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Hale ME Jr (1975) A revision of the lichen genus Hypotrachyna (Parmeliaceae) in tropical America. Smithson Contrib Bot 25:1–73
Harris RC (1993) Hypotrachyna costaricensis new to North America. Evansia 10:98
Hauck M, Javkhlan S (2009) Epiphytic lichen diversity and its dependence on bark chemistry in the northern Mongolian dark taiga. Flora 204:278–288
Jumaux G, Quetelard H, Roy D (2011) Atlas climatique de La Réunion. Météo-France, Sainte-Clotilde
Krog H, Swinscow TDV (1979) Parmelia subgenus Hypotrachyna in East Africa. Nor J Bot 26:11–43
Leavitt SD, Johnson LA, Goward T, St Clair LL (2011) Species delimitation in taxonomically difficult lichen-forming fungi: an example from morphologically and chemically diverse Xanthoparmelia (Parmeliaceae) in North America. Mol Phylogenet Evol 60:317–332
Leavitt SD, Esslinger TL, Divakar PK, Lumbsch HT (2012) Miocene divergence, phenotypically cryptic lineages, and contrasting distribution patterns in common lichen-forming fungi (Ascomycota: Parmeliaceae). Biol J Linn Soc 107:920–937
Leavitt SD, Esslinger TL, Spribille T, Divakar PK, Lumbsch HT (2013) Multilocus phylogeny of the lichen-forming fungal genus Melanohalea (Parmeliaceae, Ascomycota): Insights on diversity, distributions, and a comparison of species tree and concatenated topologies. Mol Phylogenet Evol 66:138–152
Louwhoff SHJJ, Elix JA (2002) Hypotrachyna (Parmeliaceae) and allied genera in Papua New Guinea. Bibl Lichenol 81:1–149
Lumbsch HT, Leavitt SD (2011) Goodbye morphology? A paradigm shift in the delimitation of species in lichenized fungi. Fungal Divers 50:59–72
Maddison DR, Maddison WP (2002) MacClade version 4.03PPC: analysis of phylogeny and character evolution. Sinauer Associates, Sunderland
Masson D (2005) Taxinomie, écologie et chorologie des espèces françaises des genres Hypotrachyna et Parmelinopsis (Ascomycota lichénisés, Parmeliaceae). Cryptogam Mycol 26:205–263
Masson D (2012) Hypotrachyna altorum sp. nov., a new lichen from the cloud forests of Réunion Island, Indian Ocean. Cryptogam Mycol 33:203–212
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetics trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 nov. 2010. New Orleans, Louisiana, pp 1–8
Nash TH III, Sipman HJM, Elix JA (2002) Hypotrachyna. In: Nash TH III, Ryan BD, Gries C, Bungartz F (eds) Lichen flora of the Greater Sonoran Desert Region, vol I, Lichens unlimited. Arizona State University, Tempe, pp 238–251
Normann F, Weigelt P, Gehrig-Downie C, Gradstein SR, Sipman HJM, Obregon A, Bendix J (2010) Diversity and vertical distribution of epiphytic macrolichens in lowland rain forest and lowland cloud forest of French Guiana. Ecol Indic 10:1111–1118
Online Auction Color Chart Company (2004) The online auction color chart. Palo Alto, CA
Orange A, James PW, White FJ (2010) Microchemical methods for the identification of lichens. British Lichen Society, London
Patwardhan PG, Prabhu AV (1977) Some additions to the lichen flora of India. I. Genus Hypotrachyna (Vain.) Hale (Parmeliaceae). Curr Sci 46:176–178
Poelt J (1972) Die taxonomische Behandlung von Artenpaaren bei den Flechten. Bot Notiser 125:77–81
Rambaut A (2009) FigTree v1.2.3., available at http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Suchard M, Drummond AJ (2013) Tracer v1.4, available at http://tree.bio.ed.ac.uk/software/tracer/
Rivas-Martínez S, Rivas-Sáenz S (2009) Worldwide bioclimatic classification system. Phytosociological Research Center, Madrid, Available at http://www.globalbioclimatics.org
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saipunkaew W, Wolseley PA, Chimonides PJ, Boonpragob K (2007) Epiphytic macrolichens as indicators of environmental alteration in northern Thailand. Environ Pollut 146:366–374
Sipman HJM (1993) Lichens from Mount Kinabalu. Trop Bryol 8:281–314
Sipman HJM (2002) The significance of the Northern Andes for lichens. Bot Rev 68:88–99
Sipman HJM, Elix JA, Nash TH III (2009) Hypotrachyna (Parmeliaceae, Lichenized Fungi). Flora Neotropica Monogr 104:1–176
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57:758–771
Strasberg D, Rouget M, Richardson DM, Baret S, Dupont J, Cowling RM (2005) An assessment of habitat diversity and transformation on La Réunion Island (Mascarene Islands, Indian Ocean) as a basis for identifying broad-scale conservation priorities. Biodivers Conserv 14:3015–3032
Thell A, Crespo A, Divakar PK, Kärnefelt I, Leavitt SD, Lumbsch HT, Seaward MRD (2012) A review of the lichen family Parmeliaceae—history, phylogeny and current taxonomy. Nord J Bot 30:641–664
van den Boom PPG, Brand M, Ertz D, Kalb K, Magain N, Masson D, Schiefelbein U, Sipman HJM, Sérusiaux E (2011) Discovering the lichen diversity of a remote tropical island: working list of species collected on Reunion (Mascarene archipelago, Indian Ocean). Herzogia 24:325–349
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Yánez-Ayabaca A (2009) Os gêneros Hypotrachyna e Everniastrum (Parmeliaceae, Ascomycota Liquenizados) nas províncias de Carchi e Imbabura na região Andina do Equador. Mastership dissertation, Universidade Federal do Paraná. Available at: http://dspace.c3sl.ufpr.br:8080/dspace/bitstream/handle/1884/17926/Dissertacao.Alba%20Yanez-Ayabaca.pdf?sequence=1
Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31:511–516
Acknowledgements
Field studies in Réunion were made possible with the help and advice from the Parc National de La Réunion, especially through the courtesy of Mr. B. Lequette. Field collections were made under collecting permits provided by the National Park authority. One collection of Hypotrachyna penduliloba was made during a field trip to Réunion with N. Magain; his great companionship is acknowledged here. Harrie J. M. Sipman is thanked for arranging the loan of comparative material from B and for providing nomenclatural advice. Both referees contributed to the finalization of the manuscript as their notes and suggestions were very helpful: we thank them very warmly. P.K.D. thanks the Ministerio de Ciencia e Innovación, Spain for financial support (CGL2010-21646/BOS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masson, D., Divakar, P.K. & Sérusiaux, E. Hypotrachyna penduliloba and Remototrachyna pandani, two new species in the hyperdiverse lichen family Parmeliaceae from Réunion in the Mascarene Archipelago. Mycol Progress 14, 22 (2015). https://doi.org/10.1007/s11557-015-1039-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1039-x