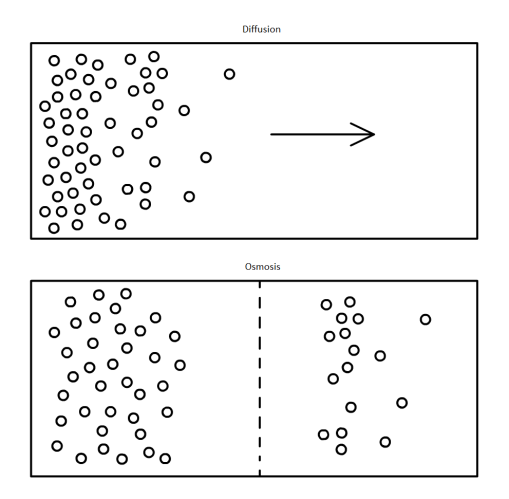
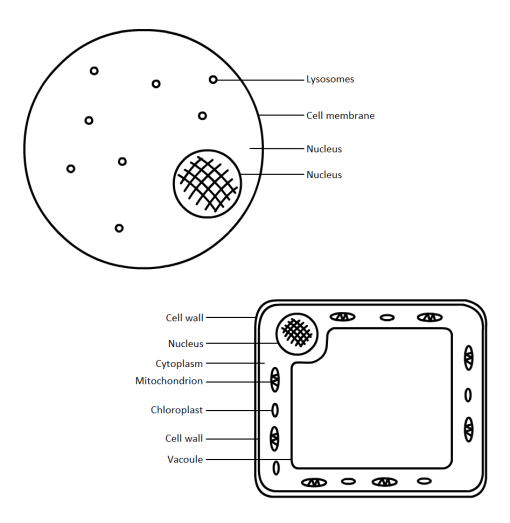
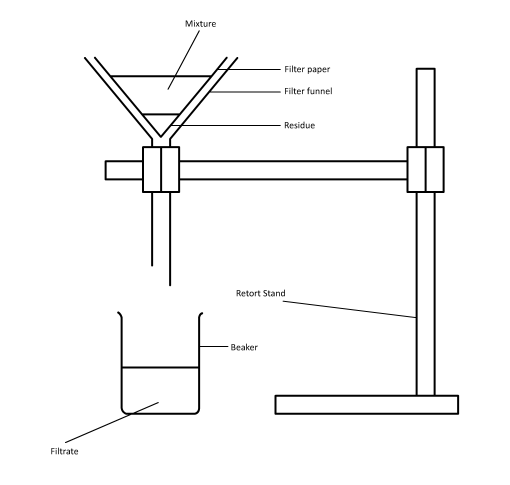
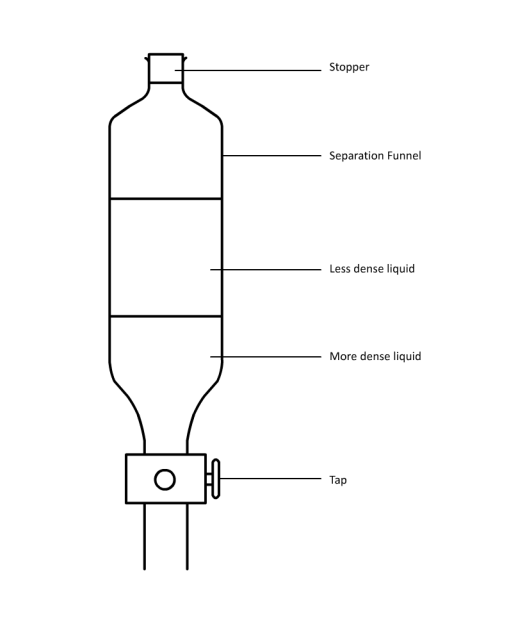
The Amaryllidaceae Hymenocallis, commonly known as the Spider Lily, is an insect-pollinated flower. The colours, being a silky white with streaks of green and orange lining the stigma and stamen, suggest that colour is the primary factor for attraction, but is instead smell.
The Hymenocallis Pedalis is pollinated by bumble bees and hummingbirds. In seeking their nectar, the aforementioned animals would brush against the stamen, releasing the pollen grains. Similarly, the animals would release the pollen grains upon contact with the stigma.