Merunggai Leaves
Moringa oleifera Lam.
Moringaceae
DEFINITION
Merunggai leaves consist of dried leaves of M.oleifera Lam. (Moringaceae).
SYNONYM
Guilandina moringa L., Hyperanthera moringa (L.) Vahl, M. zeylanica Burmann, M. pterygosperma Gaertn [ 1 , 2 ].
VERNACULAR NAMES
Horseradish-tree, ben oil tree, drumstick tree (English), merunggai, kelur, kacang kelo (Malay), la mu, lat mok (Chinese), murungai, murungai ilai (Tamil) [ 2 , 3 , 4 ].
CHARACTER
| Colour | Green (fresh leaves), dull brownish-yellow (powder) |
| Odour | Slight odour |
| Taste | Bitter, biting taste |
IDENTIFICATION
Plant Morphology
M. oleifera is a small deciduous tree or shrub, fast-growing, can grow up to 10 or 12 m in height. The tree grows with a short, straight stem that reaches a height of 1.5-2 m before it begins branching but can reach up to 3 m, extended branches grow in a disorganized manner; canopy is umbrella shaped, fragile branches, thick, corky, whitish bark that comes off in corky flakes, soft, white wood; tuberous pungent root and a thin crown; the twigs are finely hairy and green. The leaves are bipinnate or more commonly tripinnate, up to 45-60 cm long with 4-6 pairs of pinnae, and are alternate and spirally arranged on the twigs; pinnae and pinnules are opposite; leaflets are 1.2-2.0 cm long and 0.6-1.0 cm wide, the lateral leaflets elliptic, the terminal ones obovate; petioles of lateral leaflets are 1.5-2.5 mm long, those of terminal ones 3-6 mm long, the leaflets are finely hairy, and almost hairless on the upper surface, paler and hairless beneath, with red-tinged midveins, with entire margins, and are rounded or blunt-pointed at the apex and short-pointed at the base. The flowers are yellowish and fragrant, spreading or drooping axillary clusters (panicles) 10–25 cm long; individual flowers, set in a basal cup (hypanthium) ca. 3 mm long, are approximately 0.7-1 cm long and 2 cm broad, with five unequal yellowish-white, thinly veined; spathulate petals; five stamens with five smaller sterile stamens (staminodes), and a pistil composed of a 1-celled ovary and slender style. The fruits are elongated, pendulous, linear, three-sided pods with nine longitudinal ridges, usually 20-50 cm long, but occasionally up to 1 m or longer, and 2.0-2.5 cm broad. The pods, each usually containing up to 26 seeds, are dark green during their development, and take approximately 3 months to mature after flowering; turn brown on maturity, and split open longitudinally along the three angles, releasing the dark brown, trigonous seeds [ 5 , 6 , 7 ].
Microscopy
The leaves powder shows groups of spongy parenchyma, palisade cells, parenchyma with thick walled isodiametric cells and intercellular spaces; spiral xylem vessels which well developed wide vessels with reticulate thickening and shows a few bordered pits; a pieces of polyhedral epidermal cells in surface view; anomocytic stomata, present on both surface but numerous on lower surface; having multicellular uniserriate trichome, unicellular hairs with blunt tip; rosette crystals of calcium oxalate, colorless fibers in bundles of 5-10; cell containing pigment and starch [ 8 , 9 ].
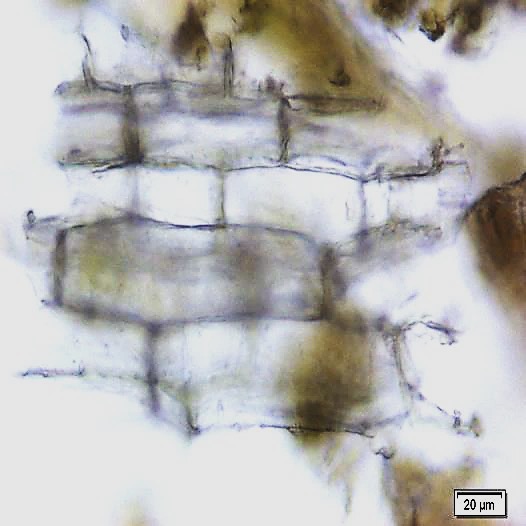

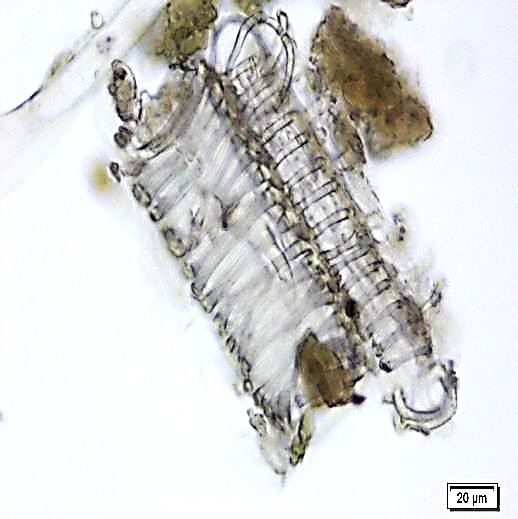
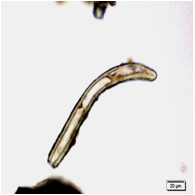


Figure 2 : Microscopic characters of M. oleifera leaf powder. (a) Epidermal cells (magnification 20x); (b) parenchyma cells (magnification 20x);(c) spiral thickening vessel (magnification 20x); (d) simple multicellular trichome (magnification 20x); (e) solitary prismatic and druse crystals (magnification 100x); (f) brachysclereid (magnification 100x). [Scale bars: a, b, c, d = 20 µm; e, f = 5 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Green |
| 5% KOH | Yellow |
| 5% FeCl3 | Dark Yellow |
Thin Layer Chromatography (TLC)
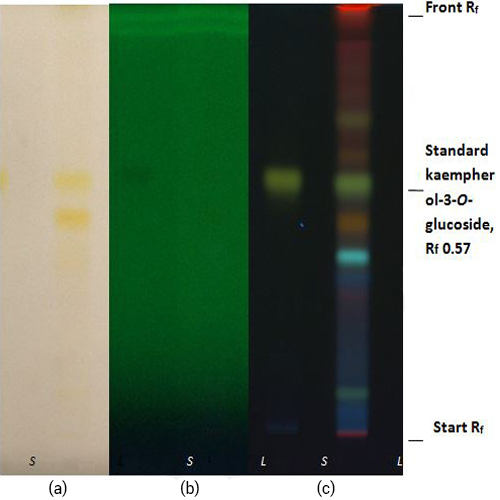
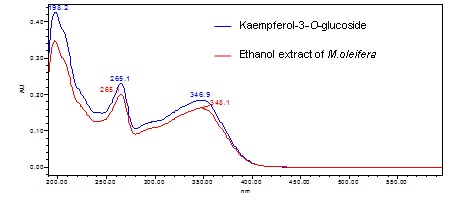
Figure 3 : TLC profiles of standard kaempherol-3-O-glucoside (S) and ethanol extract of M. oleifera dried leaf powder (L) observed under (a) at visible light (b) UV at 254 nm and (c) UV at 366 nm after derivatized with natural product reagent.
| Test Solutions | Weigh 0.5 g dried powdered leaf of M. oleifera in test tube. Add 5 mL of absolute ethanol and sonicate for 30 min at room temperature. Filter the sample and use the supernatant for TLC analysis. |
| Standard solution | Dissolve kaempferol-3-O-glucoside standard [CAS no.:480-10-4] in absolute ethanol to give 50 µg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Ethyl acetate : formic acid : acetic acid : water (34 : 3.5 : 1.5 : 7) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
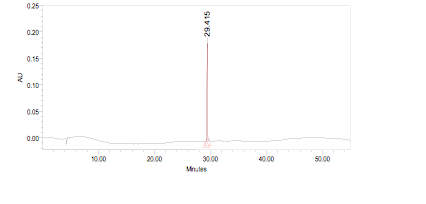
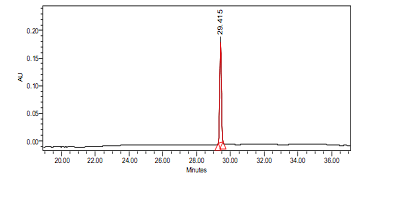
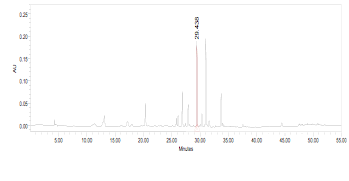
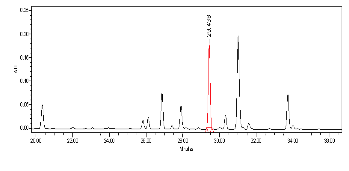
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh 0.5 g of M. oleifera dried powder in a test tube. Add 5 mL of absolute ethanol and sonicate for 30 min at room temperature. Filter the sample (Whatman No.1) and use the supernatant for HPLC analysis. Filter the mixture solution through a 0.45 µm Nylon syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||||||||
| Standard solution | Dissolve kaempferol-3-O-glucoside standard [CAS no.:480-10-4] in absolute ethanol to give 50 µg/mL solution. | ||||||||||||||||||||||||
| Chromatographic system |
Detector: PDA 347 nm |
||||||||||||||||||||||||
| Mobile Phase (Isocratic mode) |
|
||||||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the kaempferol-3-O-glucoside standard solutions (50 µg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 10% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 30% |
| Cold method | Not less than 30% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 20% |
| Cold method | Not less than 9% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Ethanol extract of the M.oleifera leaves has been reported to contain thiocarbamate glycoside [e.g. O-methyl,4-[(α-L-rhamnosyloxy benzyl]thiocarbamate (E) and (Z), O-ethyl,4-[(α-L-rhamnosyloxy benzyl]thiocarbamate (E), O-ethyl,4-[(4’-O-acetyl-α-L-rhamnosyloxy benzyl] thiocarbamate (E+Z), O-methyl,4-[(4’-O-acetyl-α-L-rhamnosyloxy benzyl]thiocarbamate (E) and (Z), O-methyl,4-[(2’,3’,4’-tri-O-acetyl-α-L-rhamnosyloxy benzyl] thiocarbamate (E), O-ethyl,4-[(α-L-rhamnosyloxy benzyl] thiocarbamate (Z); carbamate glycoside [e.g. O-ethyl,4-[(4’-O-acetyl-α-L-rhamnosyloxy) benzyl]carbamate (Z) and (E), O-methyl,4-[(2’,3’,4’-tri-O-acetyl-α-L-rhamnosyloxy benzyl]carbamate (E), O-methy,4-[(2’,3’,4’-tri-O-acetyl-α-L-rhamnosyloxy)benzyl]carbamate (Z), O-ethyl,4-[(2’,3’,4’-tri-O-acetyl-α-L-rhamnosyloxy benzyl]carbamate(E), O-methyl,4-[(4’-O-acetyl-α-L-rhamnosyloxy benzyl]carbamate (E)], benzaldehyde glycoside [e.g. 4-(4’-O-acetyl-α-L-rhamnosyloxy benzaldehyde], glycosyl [e.g. 6-[4-(cyanomethyl)phenoxy]-4,5-dihydroxy-2-methyloxan-3-yl acetate], isothiocyanate glycoside [e.g. 4-[(4’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate], glycoside [e.g. (4-(α-L-rhamnosyloxy)benzyl], phenolic glucoside [e.g. quercetin-3-O-glucoside, quercetin-3-O-(6’’-malonyl-glucoside), kaempferol-3-O-glucoside, kaempferol-3-O-(6’’-malonyl-glucoside], phenolic [e.g. 3-caffeoylquinic acid, 5-caffeoylquinic acid] [ 10 , 11 , 12 , 13 , 14 ].
Methanol extract of the M.oleifera leaves has been reported to contain alkaloid (e.g. pyrrolemarumine 4″-O-α-l-rhamnopyranoside, 4′-hydroxyphenylethanamide) [ 15 ].
Acetone extract ofthe M.oleifera leaves has been reported to contain caratenoids (e.g. neoxanthin, violaxanthin, lutein, zeaxanthin, xanthophylls and trans-β-carotene) [ 16 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
M. oleifera leaves can be used as a poultice on the abdomen to expel intestinal worms. It is also rubbed over the breasts to prevent the flow of milk. The leaves can be eaten to treat gonorrhea. The juice of the leaves is mixed with lime to treat dropsy [ 2 ]
Biological and pharmacological activities supported by experimental data
Antihyperglycemic activity
Ethanol (70%) extract of M. oleifera leaves (250 and 500 mg/kg) administered intraperitoneally to streptozocine-induced diabetic Wistar rats (20-25 weeks) significantly (p < 0.05) decrease blood glucose level (117.8 and 87.2 mg/dL) in treated rats compared to control (376.0 mg/dL) after 3 hours [ 17 ].
Aqueous extract of M. oleifera leaves (100, 200 and 300 mg/kg) was administered orally to streptozocine-induced diabetic male albino Wistar rats. A significant (p < 0.05) decrease in blood sugar level (200.7, 182.2 and 186.7 mg/dL) was observed in the sub diabetic group compared to control (217.5 mg/dL) after one hour. A significant (p < 0.05) decrease in blood sugar level (338.1, 300.9 and 307.1 mg/dL) was also observed in the mild diabetic group compared to control (375.5 mg/dL) after one hour. A significant (p < 0.05) decrease in fasting blood glucose (FBG) (≈ 210 mg/dL) and post prandial glucose (PPG) (≈ 340 mg/dL) levels were observed in severely diabetic group compared to control (FBG ≈ 330 mg/dL, PPG ≈ 450 mg/dL) after seven days [ 18 ].
Hepatoprotective activity
Ethanol (80%) extract of M. oleifera leaves (150 mg/kg body weight for five days) administered orally to high fat diet-induced male albino Swiss strain mice significantly (p < 0.05) increase in ferric reducing antioxidant power (FRAP ≈ 2.5 FU; control ≈ 1.8 FU) and reduced glutathione (GSH ≈ 1.3 mmol/mg of protein; control ≈ 0.3 mmol/mg of protein). Lipid peroxidation (≈ 1.0 nmoles of MDA/mg of protein; control ≈ 2.3 nmoles of MDA/mg of protein), aspartate aminotransferase (AST ≈ 77 IU/L; control ≈ 108 IU/L), alanine aminotransferase (ALT ≈ 71.6 IU/L; control ≈ 114.5 IU/L) and alkaline phosphatase (ALP ≈ 50 KA unit; control ≈ 84 KA unit) were also significantly (p < 0.05) decreased. Histologically, the extract was shown to conserve normal hepatic architecture compared to the high fat-diet group that shows liver damage by lipid accumulation and abnormal hepatocytes [ 19 ].
Ethanol (80%) extract of M. oleifera leaves (150 mg/kg body weight for 15 days) administered orally to high fat diet-induced male albino Swiss mice significantly (p < 0.05) increased FRAP (≈ 5.0 FU; control ≈ 1.8 FU) and GSH (≈ 1.5; control ≈ 0.3 mmol/mg of protein). Lipid peroxidation (≈0.5 nmoles of MDA/mg of protein; control ≈ 2.3 nmoles of MDA/mg of protein), AST (≈ 26.7 IU/L; control ≈ 108 IU/L), ALT (≈ 25.8 IU/L; control ≈ 114.5 IU/L), and ALP (≈ 40 KA unit; control ≈ 84 KA unit) was also significantly (p < 0.05) decreased. Histologically, the extract was shown to fully conserve normal hepatic architecture compared to the high fat-diet group [ 19 ].
Hypocholestrolaemic activity
Aqueous extract of M. oleifera leaves (0.1 g/kg/day) administered orally to high cholesterol diet-induced male adult New Zealand white rabbit for a duration of 12 weeks significantly (p < 0.05) reduce total cholesterol (TC = 714.67 mg/dL), low density lipoprotein (LDL = 671.67 mg/dL), high density lipoprotein (HDL = 174.00 mg/dL) and triglyceride (TG = 85.33 mg/dL) level after 4 weeks compared to high cholesterol diet group (TC = 1134.50 mg/dL, LDL = 1071.75 mg/dL, HDL = 265.75 mg/dL and TG = 237.25 mg/dL) [ 19 ].
Anti-atherosclerotic activity
Aqueous extract of M. oleifera leaves (0.1 g/kg/day) administered orally to high cholesterol diet-induced male adult New Zealand white rabbits reduced the internal carotid atherosclerotic percent of plaque formation (2.75%) compared to high cholesterol diet group (20.4%) after 12 weeks [ 20 ].
Antihyperthyroid activity
Aqueous extract of M. oleifera leaves (175 mg/kg body weight/day) administered orally to both male and female Swiss rats (5-6 months old) for a duration of 10 days significantly increase T4 concentration, decrease serum triiodothyronine T3 to T4 ratio and decrease the concentration of (T3) in female rats. There was no dose dependent effect when a dose of 350 mg/kg body weight/day was administered to the female rats [ 21 ].
Anti-oxidant activity
Aqueous extract of M. oleifera has total phenolic content of 205.8 ± 0.22 mg/ml gallic acid and showed anti-oxidant activity with 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals (IC50 78.15 ± 0.92 µg/mL) compared to Trolox (IC50 of 2.14 ± 0.12 µg/mL). A significant decrease in thiobarbituric acid reactive substances (TBARS) formation also observed with 2.46 ± 0.81 nmol/mg LDL protein compared to high fat diet group with 14.41 ± 5.85 nmol/mg LDL protein [ 20 ].
Aqueous extract of M. oleifera leaves has total phenolic content (105.04 ± 2.17 mg gallic acid equivalent/g), total flavonoids content (31.28 ± 1.62 mg quercetin equivalents/g), ascorbic acid content (91.22 ± 3.58 mg/100 g fresh tissues). The extract also has anti-oxidant activity (85.77 ± 5.07% compared to α-tocopherol with 73.22 ± 5.13%), antiradical power (74.3 ± 3.54 compared to α-tocopherol with 64.6 ± 3.37) and reducing power (01.1 ± 0.54 compared to α-tocopherol with 01.5 ± 0.51 ascorbic acid equivalent/mL) [ 22 ].
Acetone extract of M. oleifera leaves (1.0 mg/mL) showed anti-oxidant activity with 2,2′-azinO-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) (95.27%), DPPH (98.24%) and nitric oxide radicals (65.77%) which were comparable to butylated hydroxyltoluene (BHT) [ABTS (98.47%), DPPH (98.62%) and nitric oxide radicals (98.47%)]. Aqueous extract of the M. oleifera leaves also has percentage inhibition of ABTS (72.89%), DPPH (83.56%) and nitric oxide radicals (59.4%) which were also comparable to the BHT [ 23 ].
Ethyl acetate fraction of M. oleifera leaves (100 mg/kg) given orally to male Sprague-Dawley rats (150-200 g) significantly (p < 0.001) decreased lipid peroxidation level (≈ 0.6 mmol MDA/mg protein; CCl4 control ≈ 3.1 mmol MDA/mg protein) and increased levels of glutathione (≈ 39 µg/mg protein; CCl4 control ≈ 25 µg/mg protein), superoxide dismutase (≈ 150 U/mg protein; CCl control ≈ 75 U/mg protein) and catalase (≈ 38 U/mg protein; CCl4 control ≈ 22 U/mg protein) in the liver [ 24 ].
The extract also significantly (p < 0.001) decreased lipid peroxidation level (~0.6 mmol MDA/mg protein; CCl4 control ≈ 2.36 mmol MDA/mg protein), significantly (p < 0.01) increased levels of glutathione (≈ 29 µg/mg protein; CCl4 control ≈20 µg/mg protein), significantly (p < 0.01) increase superoxide dismutase (≈ 120 U/mg protein; CCl4 control ≈ 70 U/mg protein) and significantly (p < 0.001) increase catalase (≈ 39 U/mg protein; CCl4 control ≈ 25 U/mg protein) in the kidney [ 24 ].
Dried M. oleifera leaves (200 g/day) when fed to each castrated cross-bred Xhosa lop-eared goats (8 months old) significantly (p < 0.05) increased the activity of glutathione, catalase and superoxide dismutase activity and reduced lipid peroxidation after 60 days compared to control fed with grass hay [ 23 ].
Nephroprotective activity
Ethanol : water (80:20) extract of M. oleifera leaves (150 and 300 mg/kg/day) given intraperitoneally for 10 days to gentamicin-induced nephrotoxic adult male rabbits significantly (p < 0.01) reduce serum urea and creatinine levels compared to gentamicin control group. The extract (300 mg/kg) also caused ameliorative changes on gentamicin-induced tubular necrosis of renal [ 25 ].
Antineuropathic pain activity
Alcohol (50%) extract of M. oleifera leaves (200 mg/kg) administered orally for 21 days to streptozocin-induced diabetic male Wistar rats with neuropathic pain from chronic constriction of right sciatic nerve significantly (p < 0.05) decrease withdrawal latency (≈ 2.2 s) compared to control (≈ 1.8 s) using hot plate test at 12th day and significantly (p < 0.05) decrease withdrawal threshold (≈ 2.0 g) compared to control (≈ 0.5 g) using Von Frey test at 15th day [ 26 ].
Antimicrobial activity
Ethanol (50%) extract of M. oleifera leaves (10 g/190 mL) with quantity of 400 µL/disc inhibited Staphylococcus aureus (inhibition zone = 22.3 mm), Enterococcus faecalis (17.0 mm), Aeromonas caviae (21.2 mm) and Vibrio parahaemolyticus (17.8 mm) by using disk diffusion assay [ 27 ].
Aqueous extract of M. oleifera leaves (10 g/190 mL) with quantity of 400 µL/disc inhibited S. aureus (inhibition zone = 22.0 mm), E. faecalis (with quantity of 300 µL/disk) (16.3 mm), A. caviae (21.4 mm) and V. parahaemolyticus (20.7 mm) by using disk diffusion assay [ 27 ].
Aqueous extract of M. oleifera leaves (30 mg/mL) showed antibacterial effect to orthopaedic wound bacteria such as Klebsiella pneumoniae, Proteus vulgaris, Providencia stuartii, E. coli, Pseudomonas fluorescens, Acinetobacter baumanii, Burkholderia cepacia, Yersinia enterocolitica, Serratia rubidae, Salmonella pullorum, Klebsiella oxycota with inhibition zones ranging from 12 to 15 mm using paper disc diffusion [ 28 ].
Methanol (60%) extract of M. oleifera leaves (30 mg/mL) also showed antibacterial effect with inhibition zone ranging from 12 to 19 mm to orthopaedic wound bacteria like Streptococcus sp., P. fluorescens, A. baumanii, B. cepacia, Y. enterocolitica, Proteus mirabilis, S. rubidae, S. pullorum but did not inhibit K. pneumoniae, P. vulgaris, P. stuartii, E. coli, S. rubidae and K. oxycota [ 28 ].
Aqueous extract of M. oleifera leaves (30 mg/mL) showed antifungal activity on Candida albicans and Pullarium sp (inhibition zone = 5 mm) using paper disc diffusion. Methanol (60%) extract only inhibited the growth of Aspergillus flavus (12 mm) and ethanol (90%) extract inhibited the growth of A. flavus (15 mm), C. albicans (3 mm), Penicillium carmenberti (15 mm), Pullarium sp (20 mm) and Trichophyton mentagrophyte (22 mm) [ 28 ].
Anticonvulsant activity
Ethanol (50%) extract of M. oleifera leaves (500-2000 mg/mL) administered orally one hour prior to pentylenetetrazole-induced convulsion in male albino Swiss mice (18-25 g) produced a dose dependent protection (survival) after 24 hours [ 29 ].
Antiproliferative activity
Aqueous extract of M. oleifera leaves (200 µg/mL) significantly (p < 0.001) inhibit the proliferation of cervical cancer (KB) cell line (40% cell viability) compared to control without treatment (100% cell viability) after 48 hours of incubation using MTT assay [ 30 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Ethanol extract of M. oleifera leaves (2000-6400 mg/kg) was administered orally as a single dose to male albino Swiss mice (18-25 g). There was no toxicity effect observed for a period of 24 hours (LD50 > 6400 mg/kg) [ 29 ].
Aqueous extract of M. oleifera leaves (2000 mg/kg) was administered orally as a single dose to male Wistar rats (six weeks old). There was no toxicity effect observed for a period of 14 days (LD50 > 2000 mg/kg) [ 31 ].
Aqueous extract of M. oleifera leaves (400-6400 mg/kg) was administered orally as a single dose to male Wistar albino mice (20 g). There was no toxicity effect observed for a period of 24 hours (LD50 > 6400 mg/kg). There was a slight dullness in behaviour observed in the animals at doses above 1600 mg/kg [ 32 ].
Aqueous extract of M. oleifera leaves (400-2000 mg/kg) was administered orally as a single dose to male Wistar rats (85-130 g). There was no toxicity effect observed for a period of 48 hours (LD50 > 2000 mg/kg body weight). Rats receiving 1600 mg/kg extract and 2000 mg/kg showed slight dullness in behaviour for the first five hours but after that period, they became normal. There were death during the study period; group with 1600 mg/kg extract (one over six rats) and 2000 mg/kg extract (two over six rats) [ 33 ].
Oral single dose acute toxicity study using aqueous extract of M. oleifera leaves on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 35 ].
Sub-acute toxicity
Aqueous extract of M. oleifera leaves (400 and 800 mg/kg/day) administered orally to male Wistar rats (85-130 g) for a duration of 21 days showed significant (p < 0.05) changes in packed cell volume (PCV) (29.5-45.3%; distilled water control = 41.1%), white blood cell (WBC) (10.1-10.2×109/L; control = 9.0×109/L), lymphocytes (6.9×109/L for both doses; control = 6.3×109/L), neutrophils (2.9 x109/L for both doses; control = 2.4×109/L) and monocytes (0.3-0.5×109/L; control = 0.2×109/L). However, only dose of 800 mg/kg/day showed significant changes in haemoglobin (9.8 g/L; control = 13.4 g/L) and red blood cells (4.7×1012/L; control = 6.9 x1012/L) [ 33 ].
The aqueous extract (1600 mg/kg/day) also showed significant (p < 0.05) changes in PCV (35.0 ± 3.7%; distilled water control = 41.1 ± 3.2%), neutrophils (2.5 ± 0.2×109/L; control = 2.4 ± 0.1×109/L), haemoglobin (9.8 ± 1.3 g/L; control = 13.4 ± 1.3 g/L) and red blood cell (4.7 ± 0.4; control = 6.9 ± 0.4×1012/L), mean corpuscular values (55.3 ± 3.8 fl; control = 60.0 ± 3.0 fl) and mean cospuscular haemoglobin concentration (37.4 ± 3.5%; control = 32.6 ± 2.1%) [ 33 ].
The aqueous extracts (400-1600 mg/kg/day) also showed significant (p < 0.05) changes in total protein (5.7-7.0 g/L; distilled water control = 6.6 ± 0.1 g/L), globulin (2.4-3.8 g/L; control = 3.4 g/L), ALT (7.1-20.7 U/L; control = 14.8 U/L), AST (7.0-19.8 U/L; control = 13.2 U/L) and urea (0.1-10.2 mg/dL; control = 11.3 mg/dL). Weight gain was observed in control and all experimental groups (400, 800 and 1600 mg/kg dose). The weight gains observed within 21 days were 36.7% (dose 400 mg/kg), 27.4% (dose 800 mg/kg) and 18.1% (dose 1600 mg/kg). However, only dose of 1600 mg/kg/day showed significant changes in ALP (47.4 ±18.7 U/L; control = 28.1 ±14.5 U/L) and albumin (2.9 ±0.3 g/L; control = 3.2 ±0.4 g/L) [ 33 ].
Aqueous extract of M. oleifera leaves (2.01 g/kg/day) administered orally to male and female of Swiss albino rats (eight to ten weeks old) for a duration of 30 days showed significant (p < 0.002) changes in white blood cells (9.96 ±4.48×103 µ/L; control = 5.14 ±1.96×103 µ/L), chloride (122.71 ±11.3 mmol/dL; control = 93.34 ±9.1 mmol/dL), potassium (5.29 ±0.3 mmol/dL; control = 23.84 ±7.5 mmol/dL), calcium (2.69 ±0.22 mmol/dL; control = 0.21 ±0.62 mmol/dL), creatine phosphokinase (6564.4 ±1573 U/L; control = 501.82 ±1.79 U/L), lactate dehydrogenase (3306.3 ± 606.9 U/L; control = 508.90 ±1.79 U/L), ALP (146.75 ±37.14 U/L; control = 7.37 ±1.79 U/L) and total bilirubin (3.35 ±0.39 mg/dL; control = 0.55 ±1.79 mg/dL) [ 34 ].
The extract (16.1 g/kg/day) also showed significant (p < 0.002) changes in white blood cells (12.56 ±3.62×103µ/L; control = 5.14 ±1.96×103µ/L), chloride (124.54 ±7.7 mmol/dL; control = 93.34 ±9.1 mmol/dL), potassium (5.11 ± 0.5 mmol/dL; control = 23.84 ±7.5 mmol/dL), sodium (161.69 ±2.8 mmol/dL; control = 137.25 ±8.4 mmol/dL), calcium (3.14 ±0.7 mmol/dL; control = 0.21 ±0.62 mmol/dL), creatine phosphokinase (5389.8 ±3894.5 U/L; control = 501.82 ±1.79 U/L), lactate dehydrogenase (1791.2 ±1838.2 U/L; control = 508.90 ±1.79 U/L), ALP (110.40 ± 18.19 U/L; control = 7.37 ±1.79 U/L) and total bilirubin (4.31 ±1.07 mg/dL; control = 0.55 ±1.79 mg/dL). A significant (p < 0.002) weight gain was also observed in rats treated with 16.1 g/kg extract compared to control group. Histopathology analysis showed mild features of hepatitis, glomerulonephritis and myocarditis [ 34 ].
Reproductive toxicity
Oral aqueous extract of M. oleifera leaves (50 mg/kg) was reported to reduce oxidative stress on testicular toxicity of chromium-induced male Wistar rats (six weeks old) by reducing body weight difference (4.8 g, chromium-control group = 28 g); increasing testis weight (0.98 ± 0.4 g, chromium 0.40 ± 0.2 g), testis volume (1.0 ± 0.2 MI, chromium-control group = 0.41 ± 0.3 MI), testis weight/body weight ratio (0.005, chromium-control group = 0.002), testosterone level (1.98 ± 3.1 ng/mL, chromium-control group = 1.44 ± 0.3 ng/mL), glutathione peroxidase (0.69 ± 0.16 nmol/mg protein, chromium-control group = 0.35 ± 0.05 nmol/mg protein); and decreasing level of malondialdehyde (1.44 ±0.15 nmol/mg protein, chromium-control group = 2.51 ± 0.05 nmol/mg protein) after 100 days [ 34 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Moringa oleifera. Version 1.1; [cited on 9th December 2014]. Available from: http://www.theplantlist.org/tpl1.1/record/trO-21400003
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Vol. 2. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies, 1935; p.1495-1496.
- Musa Y, Azimah AK, Zaharah H. Tumbuhan ubatan popular Malaysia. MARDI, Serdang. 2009;p.212.
- The Ayurvedic pharmacopoeia of India, Part 1, Vol. 2. Government of India Ministry of Health and Family Welfare, Department of Ayush. 1999;p.163
- Lim TK. Edible and non medicinal plants. Vol. 3. Dordrect Heidelberg London New York: Springer. 2012;p.456-457.
- Foidl N, Makkar HPS, Becker K. The potential of Moringa oleifera for agriculture and industrial uses. Dar Es Salaam. 2001;p.1-20.
- Wan Hassan WE. Healing herbs of Malaysia. Federal Land Development Authority (FELDA). 2007;p.40.
- Prashant T, Sudhish R, Anish C, Ashish V. A pharmacognostical and phytochemical approach for study of Moringa oleifera leaves. Journal of Harmonized Research. 2012;1(1):48-59.
- Rachh PR, Dharamsi A. Pharmacognostic studies of Moringa oleifera leaflet. Pharma Science Monitor. 2012;3(3):124-133.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A. Isolation and structure elucidation of novel hypotensive agents, niazinin A, niazinin B, niazimicin and niaziminin A + B from Moringa oleifera: the first naturalIy occurring thiocarbamates. Journal of the Chemical Society, Perkin Transactions 1. 1992;3237-3241.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A. Novel hypotensive agents, niazimin A, niazimin 6,niazicin A and niazicin B from Moringa oleifera: isolation of first naturally occurring carbarnates. Journal of the Chemical Society, Perkin Transactions 1. 1994;3035-3040.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, HalidaK Aftab.Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. Journal of Natural Plants. 1994;57(9):1256-1261.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AUH. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry. 1995;38(4):957-963.
- Bennet RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L, Kroon PA. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. Journal of Agricultrual and Food Chemistry. 2003;51:3546-3553.
- Sahakitpichan P, Mahidol C, Disadee W, Ruchirawat S, Kanchanapoom T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry. 2011;72(8):791-795.
- Lakshminarayana R, Raju M, Krishnakantha TR, Baskaran V.Determination of major carotenoids in a few indian leafy vegetables by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry. 2005;53:2838-2842.
- Tende JA, Ezekiel I, Dikko AAU, Goji ADT. Effect of ethanolic leaves extract of Moringa oleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic wistar rats. British Journal of Pharmacology and Toxicology. 2011;2(1):1-4.
- Jaiswal D, Kumar Rai P, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. Journal of Ethnopharmacology. 2009;123:392–396.
- Das N, Sikder K, Ghosh S, Fromenty B, Dey S. Moringa oliefera leaf extracts prevents early liver injury and restores anti-oxidant status of mice fed with high-fat diet. Indian Journal of Experimental Biology. 2012;40:404-412.
- Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, Ratanachamnong P, Srisawat S, Pongrapeeporn KU. The in vitro and ex vivo anti-oxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. Journal of Ethnopharmacology. 2008;116:439-446.
- Tahiliani P, Kar A. Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacological Research. 1999;41(3).319-323.
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB. Oxidative DNA damage protective activity, anti-oxidant and anti-quorum sensing potentials of Moringa oleifera. Food and Chemical Toxicology. 2009;47:1109-1116.
- Moyo B, Oyedemi S, Masika PJ, Muchenje V. Polyphenolic content and anti-oxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Science. 2012;91:441-447.
- Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo anti-oxidant properties of different fractions of Moringa oleifera leaves. Food and Chemical Toxicology. 2009;47: 2196-2201.
- Ouedraogo M, Lamien-Sanou A, Ramde N, Ouedraogo AS, Ouedraogo M, Zongo SP, Goumbri O, Duez P, Guissou PI. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Experimental and Toxicologic Pathology. 2013;65:335-339.
- Khongrum J, Wattanathorn J, Muchimapura, Thukhum-mee W, Thipkaew C, Wannanon P, Tong-un T. Moringa oleifera leaves extract attenuates neuropathic pain induced by chronic constriction injury. American Journal of Applied Sciences. 2012;9(8):1182-1187.
- Peixoto JRO, Silva GC, Costa RA, Fontenelle JLS, Vieira GHF, Filho AAF, dos Fernandes Vieira RH. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pacific Journal of Tropical Medicine. 2011:201-204.
- Oluduro AO. Evaluation of antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South-Western Nigeria. Malaysian Journal of Microbiology. 2012;8(2):59-67.
- Bakre AG, Aderibigbe AO, Ademowo OG. Studies on neuropharmacological profile of ethanol extract of Moringa oleifera leaves in mice. Journal of Ethnopharmacology. 2013;149:783-789.
- Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food and Chemical Toxicology. 2011;49:1270-1275.
- Akunna G, Ogunmodede O, Saalu C, Ogunlade B, Bello A, Salawu E. Ameliorative effect of Moringa oleifera (drumstick) leaf extracts on chromium-induced testicular toxicity in rat testes. World Journal of Life Sciences and Medical Research. 2012;2:20.
- Awodele O, Oreagba I, Odoma S, Silva JA, Osunkalu V. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). Journal of Ethnopharmacology. 2012;139:330–336.
- Adedapo AA, Mogbojuri OM, Emikpe BO. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. Journal of Medicinal Plants Research. 2009;3(8):586-591.
- Kasolo JN, Bimenya GS, Ojok L, Ogwal-Okeng JW. Sub-acute toxicity evaluation of Moringa oleifera leaves aqueous and ethanol extracts in Swiss albino rats. International Journal of Medicinal Plant Research. 2012;1(6):74-81.
- Teh BP, Hamzah NF, Norazila Z, Norliyana MY, Wan Abdul Hakim WL. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2014. Report no.: HMRC 11-045/01/MO/L/N.