Rodents and Rodent Control in Hawaiʻi
Barry M. Brennan, University of Hawaii at Manoa, HAWAII INSTITUTE OF TROPICAL AGRICULTURE AND HUMAN RESOURCES
630 US ISSN 0271-9916 | NOVEMBER, 1980 | RESEARCH-EXTENSION SERIES 002
The rat and its cousin, the mouse, have been important pests to man since ancient times. They are especially important as disease carriers. They also cause enormous destruction and loss of food and property. Their control is not easy due to their ability to adapt to changes and their capacity to reproduce.
Rodents are carriers of a number of important diseases including plague, murine typhus, leptospirosis, and salmonellosis. The Hamakua coast area on the Big Island and Makawao district on Maui were former plague endemic areas, but the last reported human case occurred in 1949 (Hamakua). However, plague is still kept under surveillance by the Department of Health Vector Control Program. Although murine typhus and leptospirosis are known to occur throughout the islands, only sporadic cases have been reported.
Rodents consume, contaminate, and cause extensive damage to food and agricultural crops. For every $2.00 worth of food they eat, they cause $20.00 worth of damage. In Hawaii, state and private agencies spend more than $600,000 annually to control rodents.
There are four rodents of economic importance in Hawaii: the roof or black rat, the Norway or brown rat, the polynesian (Hawaiian) rat, and the house or field mouse. The roof rat is found in agricultural areas, wooded gulches, kiawe forests, and in both wet and dry forests. This species has now displaced the Norway rat as the most common rat found close to human habitations and especially in wet areas such as stream beds, drainage canals, and sewers. The polynesian rat is the smallest of the three rats and it apparently prefers agricultural lands, range, and wooded areas (including gulches) up to 2500 feet. Like the Norway rat, it may cause considerable damage to cane. The house mouse is smaller than the rats and is found both in urban and rural areas. On Maui, Hawaii, and Oahu, there have been cyclic mouse "out breaks" every few years. The cause is not wholly known but is probably related to an abundance of food and shelter before and during the critical breeding cycle, followed by disappearance of these essentials with the onset of drought conditions, which forces the migration of mouse populations.
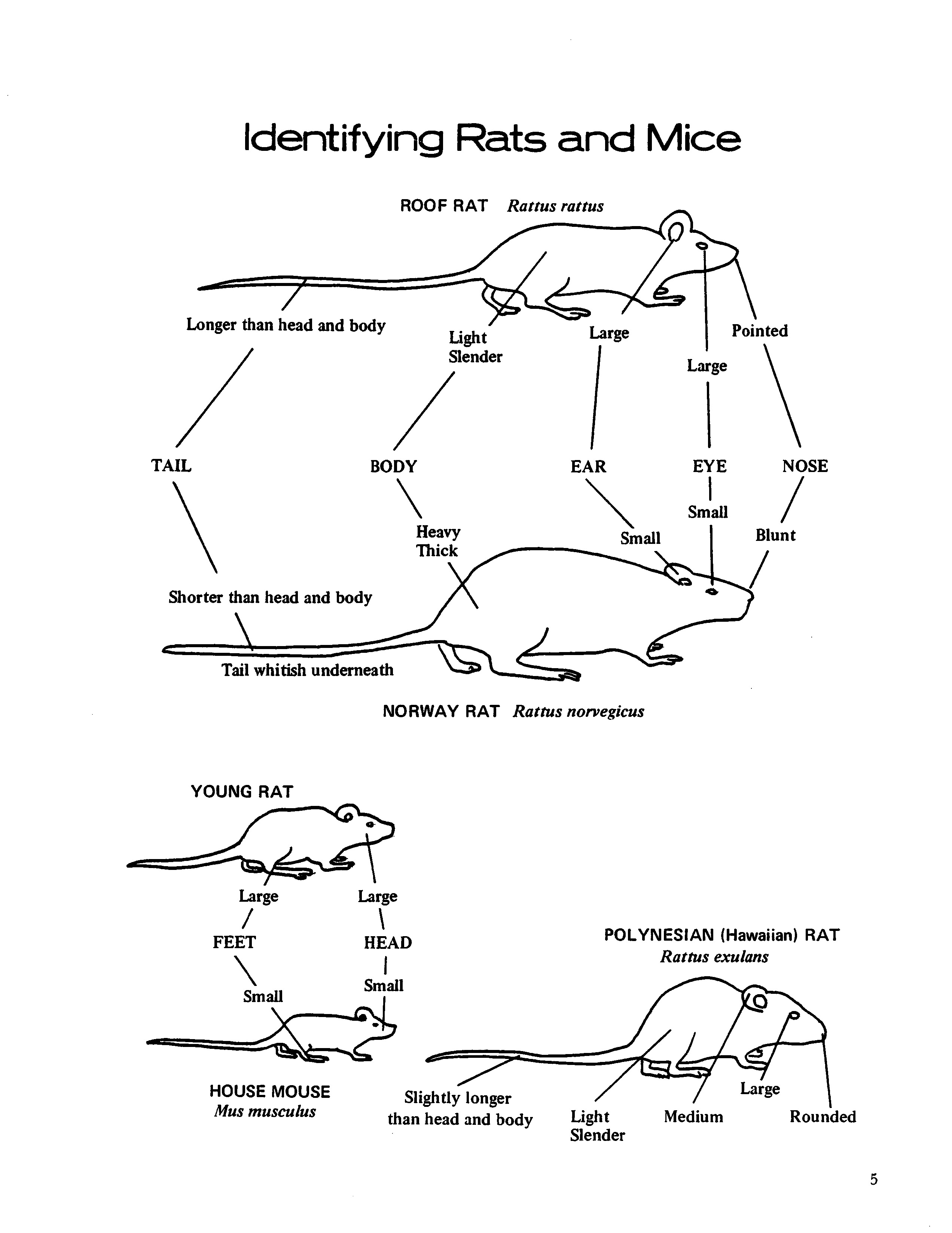
IDENTIFICATION OF RODENTS
Roof rat (Rattus rattus) - Medium to large rat, body 5 to 7 inches long. Tail slender and always longer than head and body combined. Body color varies from grey to jet black; underside grey, grey white, or white. Nose sharply pointed, large eyes, large, thin ears; in female, five pairs of nipples. Expert climber and wire scaler; frequents cane fields, macadamia nut, coffee, papaya, and banana groves; nests in attics of buildings, trees, banana bunches, and abandoned burrows of Norway rat. Moderately susceptible to plague infection.
Norway rat (Rattus norvegicus) - Largest of rats in Hawaii, weighs I0 to 18 ounces, measures
8 to 10 inches long. Tail stout, shorter than head
and body combined. Body color reddish brown to grey to black; underside whitish color. Head wide, nose blunt, ears small, eyes small, chunky in appearance; in female, six pairs of nipples. A burrowing species in ground, rubbish piles, garbage dumps, and under walks and docks; frequents sewers, pig styes, and chicken coops. May cause great damage to cane fields. Vicious. High degree of resistance to plague infection.
Polynesian rat (Rattus exulans) - Comparatively small in size, weighs 2 to 3 ounces, measures
4 to 5 inches long. Tail as long as or slightly longer than head and body combined; bristles along tail give the appearance of faint, narrow rings. Body color is cinnamon-brown to cinnamon-buff to grey; stiff black guard hairs on back and sides; underside light buff or grey. Nose roundly pointed, ears rather short, eyes medium size, hind feet dark on underside; in female, four pairs of nipples. A field rat, rarely found near buildings in Hawaii; nests in burrows, gulches, rock piles, rock walls, wastelands, fields, and embankments. Causes great damage to sugarcane, pineapple, macadamia nuts, coconuts, coffee, and other fruit and vegetable crops. Very susceptible to plague infection.
House mouse (Mus musculus) - Smallest of the
four rodent species, weighs about 1/2 ounce, measures 6 to 7 inches long from nose to tip of tail. Slender tail as long as or longer than head and body combined. Body color varies from yellowish dirty tan to dusky grey, darker over back, lighter underneath. Body slender, ears large, eyes small, nose pointed; in female, five pairs of nipples. Nests in any type of shelter, inside buildings, rock walls, rock piles, under boards, in burrows, under cane plants, and in truck-crop fields. Damage may be extensive to truck crops, flowers, etc. Degree of resistance to plague in Hawaii has not been determined.
RAT BIOLOGY
The rat is prolific. The young rat is sexually mature at four months. Sexual activity and reproductive potential are continuous until death. Rat behavior is influenced by thirst, hunger, sex, maternal instinct, and curiosity. Rats cannot go with out water for more than 48 hours or without food for more than four days. Thirsty or hungry rats be come desperate and are therefore easier to control because they are less wary. Judicious use of traps, poisons, and other control measures thus become doubly effective. Rats are nocturnal and tend to develop behavior patterns which become habitual. They have a keen sense of smell and hearing, and a fair sense of sight with ability to see in the dark.
RODENT CONTROL
Rodent control is dependent upon recognition of a rodent infestation. The most common signs are droppings, rubmarks, runways, tracks, gnawings, live or dead rats, nests, and rodent odors. Control programs must be aimed at controlling the entire population, not individual rodents. Programs must include a survey to: (1) identify the species causing the problem, (2) determine the approximate size of the population, and (3) identify the characteristics of the infected area.
Rodents establish a home range which provides food, water, shelter, and reasonable protection from predators. Cleaning up the environment by removing access to food, water, or shelter, or limiting their accessibility with physical barriers such as screens, will result in a population decline. Mechanical control achieved with the use of traps may also be important. Physical, mechanical, and environmental control should be used in conjunction with chemical control.
Rodenticides are the most effective means of controlling large and small rodent populations. However, their use entails hazards to other mammalian life, including man (especially small children), pets, and domestic animals. Some poisons have a secondary effect which may affect animals which consume dead or nearly dead rodents. Thus, it is imperative that strict safety precautions be used in the preparation, broadcast, or placement and disposal of poison baits for rodents.
Rodenticides are broadly categorized as either multi-dose or single-dose poisons. Multi-dose poisons act as sub-acute rodenticides and require repeated exposures. Rodents generally do not develop "bait shyness" to anti-coagulants. The more common sub-acute rodenticides in use are warfarin (warfacide), prolin, fumarin, pival, and diphacin.
Single-dose rodenticides act as acute poisons and include Red squill and zinc phosphide. Zinc phosphide has a pungent odor which repels pets and birds, but is attractive to rodents. Although these poisons are very effective when used properly, their toxicity and physical characteristics often place limits on their use.
After conducting a thorough rodent survey, pre-poisoning bait trials should be conducted to determine which foods and baits are most desirable to the rodent. This information and the type and location of bait containers must be recorded throughout the course of the control program. After two days of negative feeding, the bait stations should be removed and the records reviewed.
The survey should be repeated in about two months, the approximate interval for a second generation. Properly utilized environmental and physical controls will prevent rapid infestation and population build-up.
REFERENCE
Watanabe, Walter. Rodents. In: Vector Control Training Manual Chapter XIV. 1975.
PRINT VERSION of this webpage