Uca (Gelasimus) jocelynae, Shih, Hsi-Te, Naruse, Tohru & Ng, Peter K. L., 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.193214 |
|
DOI |
https://doi.org/10.5281/zenodo.5668460 |
|
persistent identifier |
https://treatment.plazi.org/id/7560B859-FFEF-6A02-04D0-D58EFA895A66 |
|
treatment provided by |
Plazi |
|
scientific name |
Uca (Gelasimus) jocelynae |
| status |
sp. nov. |
Uca (Gelasimus) jocelynae View in CoL sp. nov.
( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Mesuca (Latuca) neocultrimana Bott 1973: 317 View in CoL [part; Halmahera in Sulawesi].
Uca marionis View in CoL — Takeda & Yamaguchi 1973: 18 [double-clawed male from Palau].
Uca (Thalassuca) vocans pacificensis Crane 1975: 90 View in CoL [part; Ambon in Indonesia, Sulu, Zamboanga, and Davao in Philippines, Madang in New Guinea, Guam].
Uca vocans pacificensis View in CoL — Barnwell 1982: 70 –83 [part; Palau, Guam]; Yamaguchi, 1994: 185 [Ishigaki in southern Ryukyus].
Uca vocans vocans View in CoL — Tzeng & Chen 1992: 163 [Taitung in eastern Taiwan].
Uca borealis View in CoL — Ho et al. 1993: 20 [part?; Taitung in eastern Taiwan]; Lee 2001: 102 [part; photo taken from Tainan in southwestern Taiwan]; Ng et al. 2001: 37 [part]
Uca neocultrimana — Takeda 1995: 104 [Ishigaki and Iriomote in southern Ryukyus].
Uca View in CoL ( vocans View in CoL ) borealis View in CoL — Jeng 1998: 95 [part; Kenting (= Kending) in southern Taiwan]; Shih 1998: 67 [part; photo taken from Kenting in southern Taiwan].
Uca vocans View in CoL – Minemizu 2000: 311 [photo taken from Kume I. in central Ryukyus].
Uca (Gelasimus) neocultrimana — Ng et al. 2008: 240 [part].
Type material. Holotype: male (21.7 × 13.7 mm) (NMNS-6177-001), Cingluo, Penghu, Taiwan, coll. Hsi-Te Shih, 26 Jun. 2006. Paratypes: 1 male (20.0 × 12.7 mm) (NCHUZOOL 13301), Chihsi, Penghu, Taiwan, coll. H.-T. Shih, 19 May 2007; 10 males (17.1 × 11.3 – 23.9 × 15.4 mm) (NCHUZOOL 13308), 3 males (18.4 × 11.8 – 22.2 × 14.2 mm) ( ZRC 2009.0924), 3 males (19.2 × 12.3 – 22.4 × 14.1 mm) (RUMF-ZC-1075), Chihsi, Siyu, Penghu, Taiwan, coll. H.-T. Shih et al., 19 Aug. 2009; 5 males (11.9 × 7.5 – 21.1 × 13.5 mm) (NCHUZOOL 13306), Cingluo, Penghu, Taiwan, coll. H.-T. Shih et al., 18 Aug. 2009; 2 males (14.4 × 9.5, 19.5 × 12.4 mm) (NCHUZOOL 13307), Shuanhu, Caiyuan, Penghu, Taiwan, coll. H.-T. Shih et al., 19 Aug. 2009; 2 males (22.0 × 13.6, —× 9.7 mm) (NCHUZOOL 13299), Shihcyuan, Penghu, Taiwan, coll. H.-T. Shih et al., 27 Jun. 2006. Other material examined. Japan: 4 males (15.1 × 9.8 – 19.7 × 12.6 mm), 2 females (19.6 × 13.0, 22.0 × 14.9 mm) (RUMF-ZC-1077), Fukido R., Ishigaki Island (= I.), southern Ryukyus, coll. T. Naruse, 31 Jul. 2009; 8 males (11.8 × 7.7 – 19.1 × 12.6 mm), 6 females (9.5 × 6.3 – 18.6 × 13.0 mm) (RUMF- ZC-1076), Geda R., Iriomote I., southern Ryukyus, coll. T. Naruse, 16 Aug. 2009; 2 males (11.7 × 7.3, 17.5 × 11.0 mm), 2 females (12.2 × 7.9, 15.1 × 9.7 mm) (RUMF-ZC-1080), Funaura Bay, Iriomote I., southern Ryukyus, coll. T. Naruse, 24 Apr. 2009; 1 male (18.2 × 11.5 mm) ( TMCD CHCD 678), Shira R., Iriomote I., southern Ryukyus, coll. H.-C. Liu, 11 Jul. 1995; 1 male (19.4 × 12.0 mm) ( TMCD CHCD 686), Nishida R., Iriomote I., southern Ryukyus, coll. H.-C. Liu, 12 Jul. 1995; 3 males (20.2 × 12.6 – 20.9 × 13.5 mm) (NCHUZOOL 13300),?Iriomote I., southern Ryukyus, coll. M. Salim; 1 male (20.4 × 12.5 mm) ( TMCD), Iriomote I., southern Ryukyus, coll. K. Wada, 17 Oct. 1982. Taiwan: 1 male (14.2 × 9.5 mm) (NCHUZOOL 13298), Magang, Taipei County, coll. J.-H. Lee, 13 Sep. 2003; 2 males (17.6 × 11.5, 18.7 × 12.0 mm) (NCHUZOOL 13171), Ilan, coll. S. Huang, 31 May 1996; 2 males (15.5 × 9.6, 21.2 × 13.1 mm) ( TMCD CHCD 792), Dulanwan, Taitung, coll. H.-C. Liu, 22 Apr. 1994; 4 males (17.4 × 11.1 – 20.2 × 12.7 mm) ( NTOU), Dulanwan, Taitung, coll. P.-H. Ho, 7 Apr. 2001; 3 males (13.9 × 8.9 – 14.8 × 9.2 mm), 1 female (13.1 × 9.0 mm) ( TMCD CHCD 749), estuary of Baoli River (= R.), Checheng, Pingtung, coll. H.-C. Liu, 2 Aug. 1995; 1 male (12.3 × 8.1 mm) ( TMCD CDCD 474), Houwan, Checheng, Pingtung, coll. H.-C. Liu, 2 Aug. 1994; 1 male (11.1 × 7.4 mm) ( TMCD N.0038), Wanlitong, Pingtung, coll. C.-H. Wang, 10 Feb. 1993; 2 males (12.0 × 7.7, 13.3 × 8.4 mm) ( TMCD 930210), Wanlitong, Pingtung, coll. C.-H. Wang, 10 Feb. 1993. 2 males (19.3 × 12.1, 19.7 × 12.5 mm) (NCHUZOOL 13302), estuary of Yanshuei R., Tainan City, coll. J.-H. Lee et al., 4 Aug. 2009. Philippines: 3 males (10.6 × 7.3 – 13.3 × 8.8 mm) ( ZRC 2009.0926), near San Vicente port (18°30.611’N, 122°09.185’E), Municipality of Sta. Ana, Cagayan Province, muddy intertidal, rocky shore, coll. J. C. E. Mendoza & T. Naruse, 21 Apr. 2007; 2 males (15.5 × 10.0, 16.0 × 10.2 mm) ( ZRC 2009.0927), mangroves beside provincial highway (18°29.502’N, 122°09.244’E), Municipality of Sta. Ana, Cagayan Province, muddy intertidal, rocky shore, coll. J. C. E. Mendoza & T. Naruse, 23 Apr. 2007; 6 males (15.6 × 10.0 – 18.4 × 11.5 mm), 3 females (12.1 × 8.1 – 19.7 × 13.4 mm), 2 ovigerous females (11.1 × 7.7 – 18.0 × 12.3 mm) ( ZRC 2009.0925), 2 males (11.9 × 7.5, 18.9 × 12.1 mm), 1 female (20.9 × 14.7 mm) ( NMCR), inside lagoon near Doljo Pt., Panglao I., Bohol, Panglao Marine Biodiversity Project stn. M9 (9°35.1’N, 123°43.6’E), muddy sand flat with seagrass, fringe mangroves, 0.5 m, coll. Panglao Marine Biodiversity Project, 4 Jun. 2004; 5 males (11.7 × 7.3 – 17.1 × 10.8 mm) ( MNHN), Danao, Panglao I., Bohol, Panglao Marine Biodiversity Project stn. M3 (09°32.5’/ 09°33.1’N, 123°44.7’/ 123°45.5’E), intertidal to shallow subtidal reef, 0–2.5 m, coll. Panglao Marine Biodiversity Project, 1 Jun. 2004; 2 males (10.6 × 7.0, 12.3 × 8.0 mm) (NCHUZOOL 13176), 1 male (16.1 × 10.2 mm) (NCHUZOOL 13177), Camiguin I. mangroves, 31 Aug. 2003. Indonesia: 2 males (12.3 × 7.8, 13.1 × 8.4 mm) ( ZRC 2009.0928), mangroves beside beach cottage, Bunaken, North Sulawesi, coll. N. K. Ng & C. Y. Lai, 18 Sep. 2003; 3 males (14.5 × 9.4 – 17.1 × 10.9 mm) ( ZRC 2009.0929), mangroves beside beach cottage, Bunaken, North Sulawesi, 23 Sep. 2003. Papua New Guinea: 3 males (14.4 × 9.1 – 16.8 × 10.8 mm) (QM W26812), Bootless Bay, Loloata I., coll. N. Coleman, 21 Jul. 1998. Vanuatu: 1 male (16.1 × 10.8 mm) ( ZRC 2009.0930), vicinity of Luganville, Segond Channel, Santo, stn. VM53 (15˚31’S, 167˚11.9’E), intertidal, soft and hard bottom, coll. Santo Marine Biodiversity Survey, 6 Oct. 2006.
Comparative material. Uca neocultrimana ( Bott, 1973) . 1 male (28.0 × 17.2 mm) (SMF-5654, holotype of Mesuca (Latuca) neocultrimana Bott, 1973 ), Viti, Fiji; 1 male (17.0 × 12.0 mm) ( USNM 137670, holotype of Uca (Thalassuca) vocans pacificensis Crane, 1975 ), Suva, Viti, Fiji, coll. J. Crane, Jul. 1956 (examined by J. C. E. Mendoza); 1 male (18.2 × 11.9 mm) (SMF-34746), mangrove area, Suva, Viti, Fiji, coll. R. Diesel, 30 Nov. 1997; 1 male (18.0 × 11.2 mm) (NCHUZOOL 13303), stn. 29, Pointe Utu, Wallis, coll. J. Poupin & M. Juncker, 23 Feb. 2009; 1 male (12.9 × 8.5 mm) ( MNHN), stn. 24, Halalo mangroves de Halalo, near gas terminal, Wallis, coll. J. Poupin & M. Juncker, 23 Oct. 2007; 1 female (12.8 × 8.3 mm) ( MNHN), stn. 29, Pointe Utu, Wallis, coll. J. Poupin & M. Juncker, 25 Oct. 2007.
Uca vocans (Linnaeus, 1758) . China: 12 males (13.1 × 8.9 – 26.3 × 17.3 mm), 2 females (15.9 × 11.3, 16.1 × 11.3 mm), 2 ovigerous females (14.6 × 10.2, 15.5 × 11.0 mm) (NCHUZOOL 13182), Yalong Bay, Sanya, Hainan, China, coll. H.-T. Shih & J.-H. Lee, 28 Jun. 2004. Japan: 1 male (21.0 × 14.0 mm) (RUMF- ZC-01079), Fukido R., Ishigaki I., southern Ryukyus, coll. T. Naruse, 31 Jul. 2009; 6 males (15.0 × 10.3 – 22.6 × 15.6 mm), 1 female (19.6 × 13.7 mm) ( TMCD CHCD 695), Nagura Bay, Ishigaki I., southern Ryukyus, coll. H.-C. Liu, 13 Jul. 1995; 2 males (15.9 × 11.2 – 16.0 × 11.0 mm), 1 female (19.0 × 13.3 mm) ( TMCD CHCD 717), Ishigaki I., southern Ryukyus, coll. H.-C. Liu, 13 Jul. 1995; 1 male (16.0 × 10.9 mm) (RUMF- ZC-01078), Geda R., Iriomote I., southern Ryukyus, coll. T. Naruse, 16 Aug. 2009; 1 male (18.9 × 12.3 mm), 2 ovigerous females (14.7 × 10.2, 15.0 × 10.1 mm) (RUMF-ZC-01081), Funaura Bay, Iriomote I., southern Ryukyus, coll. T. Naruse, 24 Apr. 2009; 1 male (22.1 × 14.8 mm) ( TMCD), Iriomote I., southern Ryukyus, coll. K. Wada, 17 Oct. 1982. Philippines: 1 male (19.7 × 13.3 mm) ( ZRC 2009.0932), Tambobog, Santhu, Negros Oriental, Philippines, coll. N. K. Ng et al., 6 Jul. 2004; 1 male (19.2 × 13.0 mm) ( ZRC 2009.0931), inside lagoon near Doljo Pt., Panglao I., Bohol, Panglao Marine Biodiversity Project stn. M9 (9°35.1’N, 123°43.6’E), muddy sand flat with seagrass, fringe mangroves, 0.5 m, coll. Panglao Marine Biodiversity Project, 4 Jun. 2004; 4 males (19.5 × 13.1 – 22.6 × 15.1 mm), 1 female (14.4 × 9.5 mm) (NCHUZOOL 13167), Zamboanga, Mindanao, Philippines, 10 Jun. 2006. Singapore: 1 male (16.7 × 11.5 mm) (NCHUZOOL 13189), Lim Chu Kang mangroves, coll. H.-T. Shih, 23 Aug. 2003. Indonesia: 1 male (19.4 × 13.6 mm) ( ZRC 2009.0933), Kuta, Lombok, coll. Z. Jaafar & A. Anker, 11 Feb. 2002.
Uca borealis Crane, 1975 View in CoL . Japan: 2 males (23.4 × 15.5, 24.1 × 16.1 mm) (NCHUZOOL 13297), Hitotsuba Inlet, Miyazaki Prefecture, Kyushu, coll. H. Suzuki, 11 Oct. 2008. Taiwan: 1 male (26.5 × 17.7 mm) (NCHUZOOL 13170), Ilan, coll. S. Huang, 31 May 1996; 7 males (14.4 × 9.9 – 27.7 × 18.7 mm), 5 females (13.6 × 9.7 – 25.1 × 17.1 mm) (NCHUZOOL 13220), Cigu, Tainan, coll. H.-T. Shih, 31 May 1996; 1 male (26.9 × 18.5 mm) (NCHUZOOL 13178), Cingluo, Penghu, coll. H.-T. Shih, 15 Aug. 2006. Hong Kong: 4 males (17.0 × 11.6 – 22.7 × 15.1 mm) 4 females (17.0 × 11.8 – 19.6 × 13.5 mm) (NCHUZOOL 13207), Starfish Bay, coll. B. K. K. Chan, Jul. 2004.
Uca vomeris McNeill, 1920 View in CoL . New Caledonia: 1 male (12.1 × 8.6) (MNHN), stn. 9, Presqu'île de Pindaï, coll. J. Poupin & M. Juncker, 12 Mar. 2009.
Diagnosis. Carapace trapezoidal, CW 1.43–1.63 times CL (mean = 1.55, n = 62). Lateral margins straight, divergent anteriorly, external orbital angle sharp, directed laterally. Front narrow, base of front constricted. Infraorbital margin visible in dorsal view, lined with rectangular teeth; floor of orbit smooth, without crest.
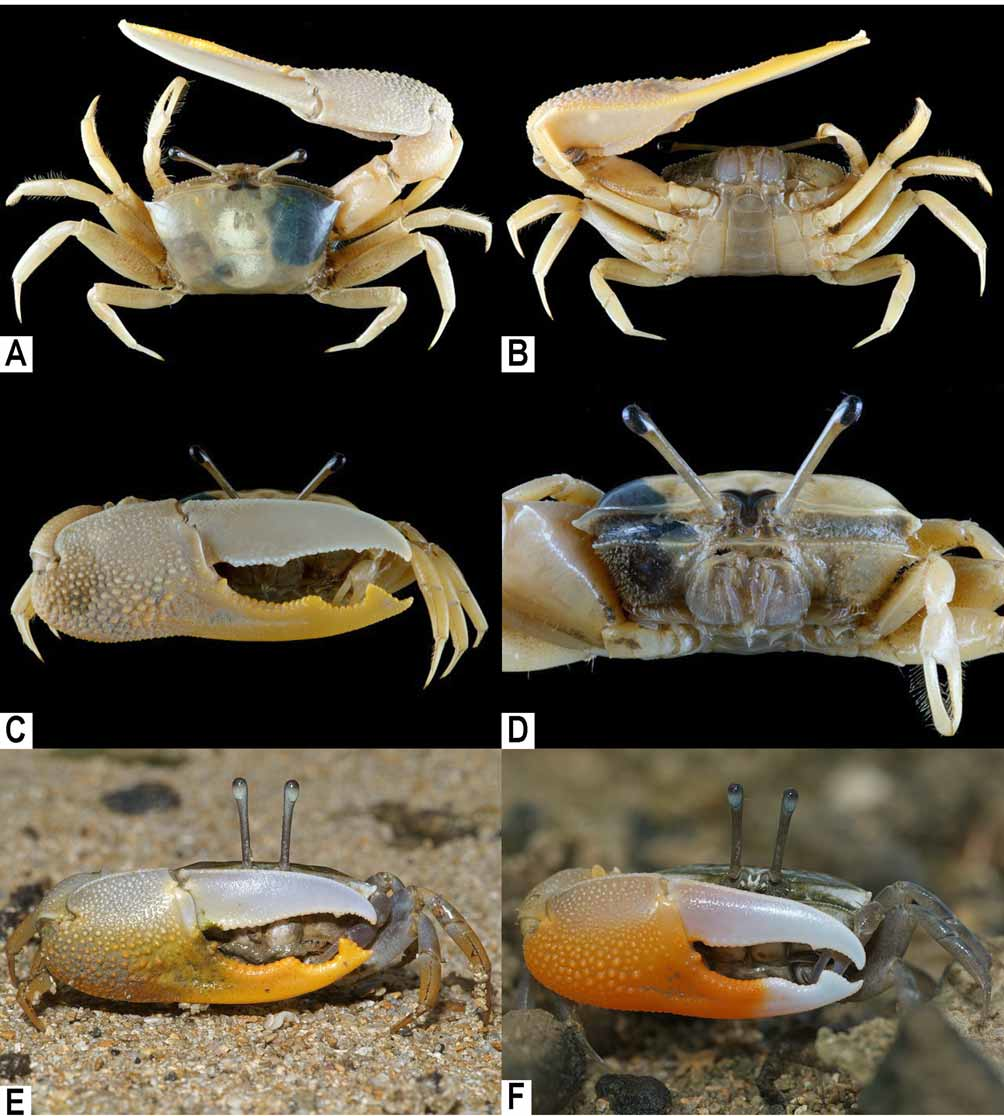
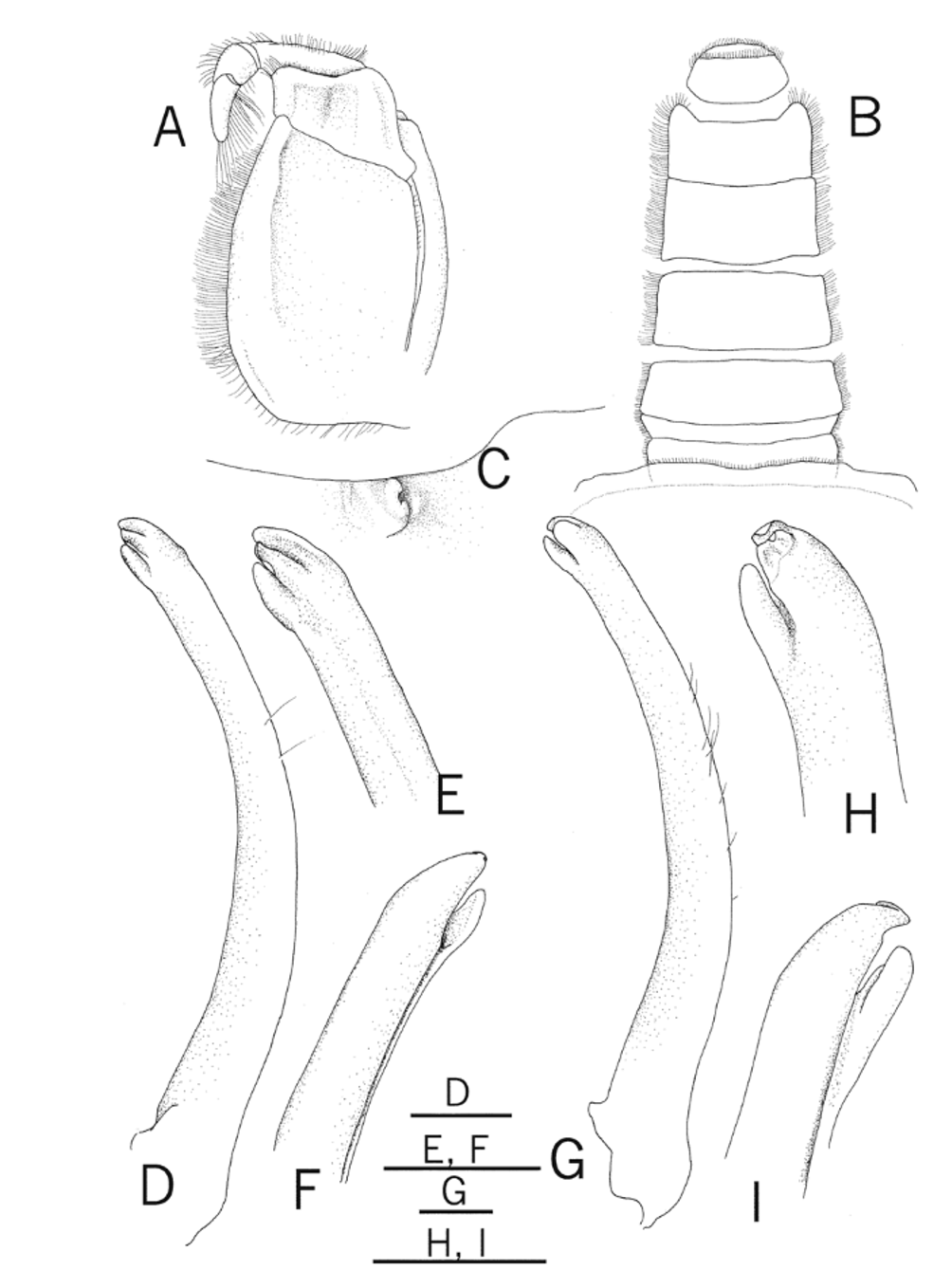
Third maxilliped ( Fig. 3 View FIGURE 3 A) rectangular, midlength of ischium about 4 times length of merus. Male major cheliped with subdistal tooth on anterior margin of merus; chela ( Fig. 2 View FIGURE 2 C, E, F) with palm granular at outer surface, inner surface with oblique row of granules proximoventrally; movable finger wide, widest point slightly wider than counterpart of immovable finger, gradually tapering distally, without groove on outer surface; immovable finger with 2 distinct tooth on distal half, with short groove on outer surface. Meri of ambulatory legs subrectangular in cross-section. G1 ( Fig. 3 View FIGURE 3 D–F) relatively long, slender, entire length gently curved, with constant diameter throughout; distal tip trilobate, lateral lobe thin, corneous, ventral lobe digitiform. Female vulva ( Fig. 3 View FIGURE 3 C) just below suture between thoracic sternites 5 and 6, with rounded opening, directed mesially.
Etymology. This species is named after Jocelyn Crane, whose landmark work on Uca ( Crane 1975) remains a masterpiece of synthetic taxonomy. Her substantial contributions to carcinology were reviewed in depth by Boyko (2000).
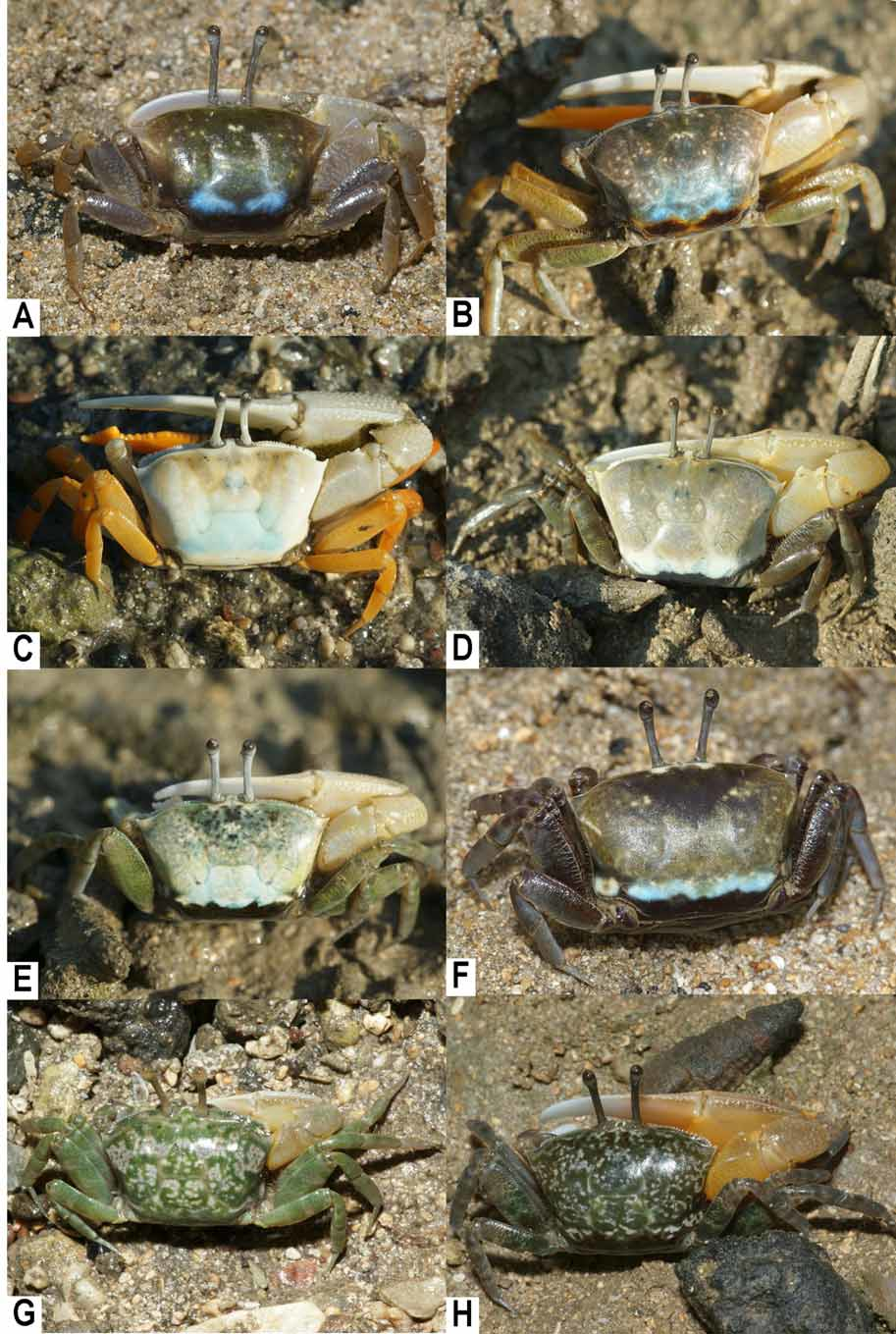
Coloration. The carapace of adults is white or whitish gray to dark brown, often with a light blue cardiac region ( Fig. 4 View FIGURE 4 A–F). The lower half of the palm and immovable finger of the major cheliped are deep yellow to orange ( Fig. 2 View FIGURE 2 E, F). The ambulatory legs are whitish gray or orange to brown ( Fig. 4 View FIGURE 4 A–H). The carapaces of small individuals sometimes have olive background with white patches ( Fig. 4 View FIGURE 4 G, H).
Ecological notes. The habitat of U. jocelynae sp. nov., is usually near river mouths of islands with fringing coral reefs. The new species appears to prefer substrate of coarse sands with slight mud, but it can be sometimes found around somewhat muddy estuaries, occasionally with mangroves nearby. This species is sympatric with U. vocans , U. borealis , U. vomeris , U. tetragonon (Herbst, 1790) , U. coarctata (H. Milne Edwards, 1852) , U. dussumieri (H. Milne Edwards, 1852) , U. crassipes (White, 1847) , U. lactea (De Haan, 1835) , U. perplexa (H. Milne Edwards, 1837) , U. triangularis (A. Milne-Edwards, 1873) ( Crane 1975; Tzeng & Chen 1992; Ho et al. 1993; Yamaguchi 1994; this study).
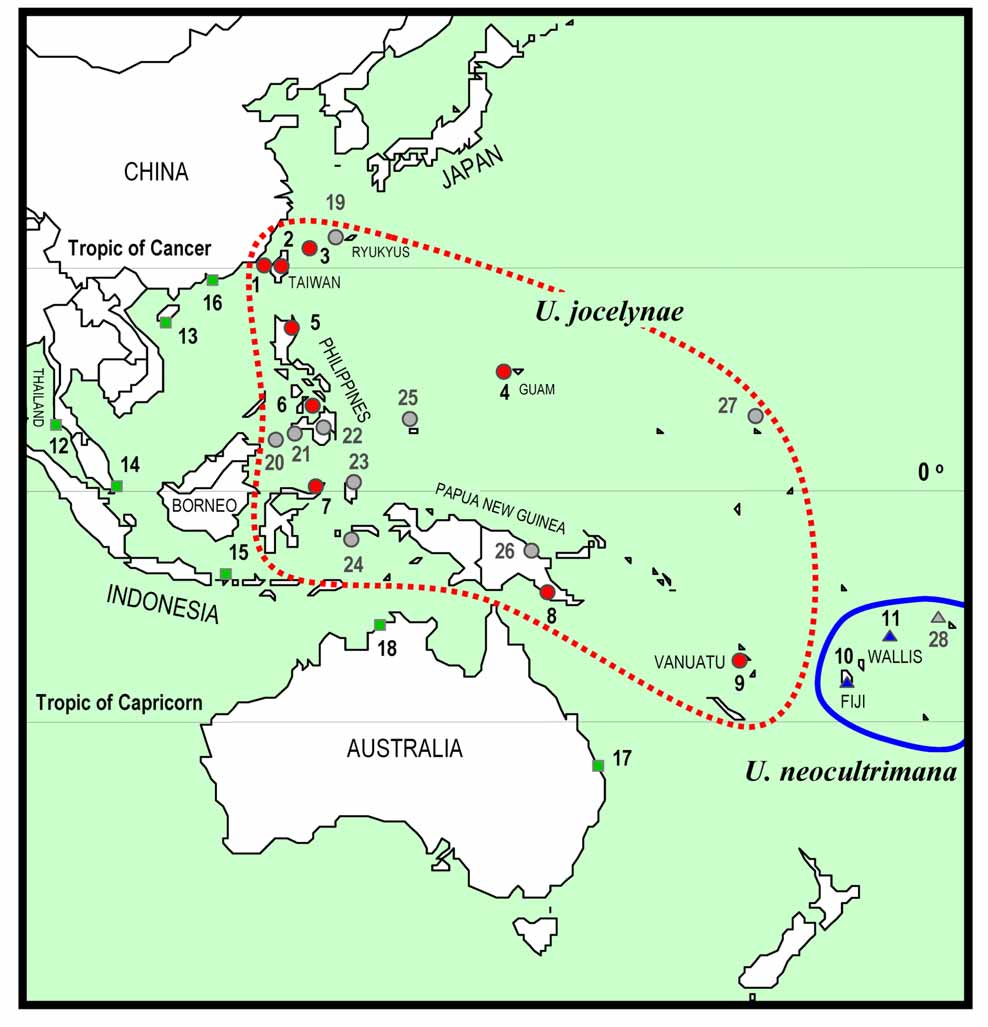
Distribution. This species is widely distributed in the Western Pacific islands, including the Ryukyus (e.g., Ishigaki and Iriomote islands), Taiwan, Guam, the Philippines, Sulawesi, Papua New Guinea, and Vanuatu. In Taiwan, this species is found from northern (Magang, Taipei County), eastern (Ilan and Taitung), southern (Pingtung), and southwestern parts (Tainan City) of the main island; and the offshore Penghu Islands (Pescadores) in the Taiwan Strait ( Fig. 1 View FIGURE 1 ).
Remarks. Uca jocelynae sp. nov. is morphologically similar to U. neocultrimana ( Bott, 1973) [type locality: Fiji] ( Fig. 1 View FIGURE 1 ). The new species can, however, be distinguished from U. neocultrimana by the characters of the male major chela and the shape of the carapace. The proximal part of the movable finger of the male major chela is only slightly wider than the counterpart of the movable finger; the two teeth of the immovable finger are more distinct and have a relatively deep gap between them ( Fig. 2 View FIGURE 2 C, E, F); with the external orbital angle directed anterolaterally ( Figs. 2 View FIGURE 2 A, 3A–H). In contrast, U. neocultrimana has the proximal part of the movable finger of the male major chela clearly wider than the same area of the movable finger; two teeth of the immovable finger are proportionately smaller and only has a shallow gap between the teeth ( Fig. 5B View FIGURE 5. A, B , D); with the external orbital angle directed anteriorly ( Fig. 5A View FIGURE 5. A, B , C). There is also a distinct difference in the shape of the vulvae, or female gonopore. In U. jocelynae sp. nov., the vulva is round, shaped like a doughnut and directed mesially ( Fig. 3 View FIGURE 3 C); while in U. neocultrimana , the opening of the vulva consist of two lobes ( Crane 1975: Fig. 64BB).
Crane (1975) described U. vocans pacificensis from Viti Levu, Fiji. The carapace ( Fig. 5 View FIGURE 5. A, B C), the major chela ( Fig. 5 View FIGURE 5. A, B D), and the G1 of the holotype of U. pacificensis are identical with U. neocultrimana s. str., and we agree that both names are subjective synonyms. The holotypes of both U. neocultrimana and U. pacificensis were collected from Fiji. Crane (1975) also regarded specimens from the Western Pacific islands (the Philippines, New Guinea, Marshall Is., and Guam) ( Fig. 1 View FIGURE 1 ) as U. pacificensis . Our examination of specimens from these islands (except the Marshall Is. and Samoa), however, shows that they are actually U. jocelynae sp. nov. (see below). It is thus highly possible that that Crane’s (1975) material from localities other than Fiji and Samoa ( Fig. 1 View FIGURE 1 ) are all U. jocelynae .
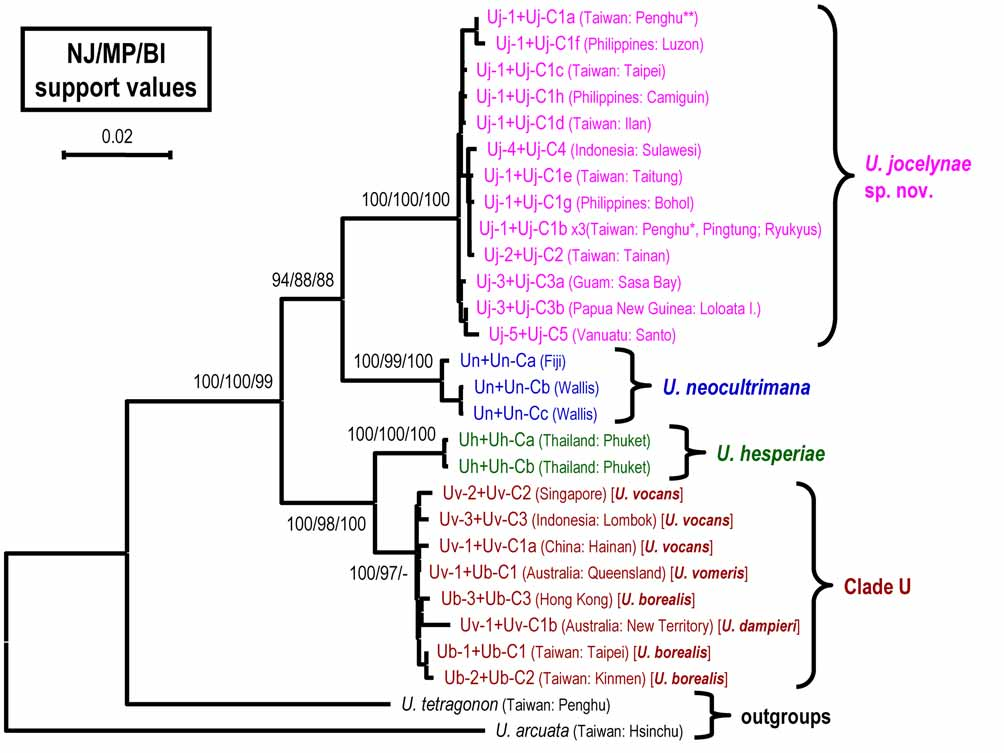
DNA analysis. A 526-bp segment (excluding the primer regions) of 16S rRNA from 28 specimens (excluding the outgroups) was amplified and aligned. Of these, 37 positions were variable and 23 parsimoniously informative, and 13 different haplotypes were distinguished ( Table 1). The studied segment of the 16S rRNA sequences was AT rich (69.2%) (T, 35.2%; A, 34.0%; G, 19.6%; C, 11.2%). For the COI gene from 28 specimens, a 658-bp segment was compared, resulting in 24 different haplotypes ( Table 1). The studied segment of the COI sequences was also AT rich (62.7%) (T, 34.4%; A, 28.3%; G, 17.2%; C, 20.0%). In this gene fragment, 90 positions were variable and 77 were parsimoniously informative. The best model selected by MrModeltest for the combined 16S rRNA and COI datasets was HKY+I+G model (TRatio=12.1709, proportion of invariable sites = 0.7066, gamma distribution shape parameter = 1.0695). The phylogram of the 1193-bp combined dataset constructed from the partitioned BI analysis, with the posterior probability and bootstrap values from the BI and MP analyses, is shown in Figure 6 View FIGURE 6. A . Only values> 50% are shown. For the MP analysis, a single tree was recovered with a tree length of 367 steps, a consistency index of 0.80, and a retention index of 0.91.
Based on the phylogenetic tree of combined datasets ( Fig. 6 View FIGURE 6. A ), the Uca vocans complex is monophyletic with high support by three methods. Four strongly supported clades can be discerned. Three clades are composed of U. jocelynae sp. nov., U. neocultrimana , and U. hesperiae , respectively. However, U. borealis , U. dampieri , U. vocans , and U. vomeris form an unsolved clade (designated here as “clade U” for convenience). Trees derived from just 16S rRNA or COI also show similar topologies (not shown).
The pairwise nucleotide divergences for 16S rRNA and COI (in parentheses) with K2P distance and differences in the total bp numbers (gaps considered) are shown in Table 2 View TABLE 2 . The mean interspecific 16S rRNA (COI) K2P distance of U. jocelynae sp. nov. is 3.41% (5.32%) with the closest U. neocultrimana which is 9.8 (8.7) times greater than the mean intraspecific distance of U. jocelynae , 0.35% (0.61%) ( Table 2 View TABLE 2 ). In addition, the lowest interspecific 16S rRNA (COI) K2P distance of U. jocelynae is 3.16% (4.94%) with U. neocultrimana which is 5.5 (4.0) times greater than the largest intraspecific distance of U. jocelynae , 0.58% (1.23%).
Within clades Between clades
Uca neocultrimana ( Bott, 1973) (= U. pacificensis Crane, 1975 View in CoL ), was considered to be widely distributed in many islands of Southeast Asia, Oceania ( Bott 1973; Crane 1975; Barnwell 1982), and East Asia (the Ryukyus) ( Yamaguchi 1994; Takeda 1995; Yoshigou 2001). Our morphological and molecular studies, however, revealed that what has been referred to as U. neocultrimana actually contains two distinct allopatric species: one from Fiji and Wallis; and the other from Western Pacific islands to the west of Fiji, including the Ryukyus, Taiwan, the Philippines, Indonesia, Papua New Guinea, and Vanuatu ( Figs. 1 View FIGURE 1 , 6 View FIGURE 6. A ). Since the holotypes of both U. neocultrimana and U. pacificensis View in CoL are conspecific (see Remarks), a new species, U. jocelynae View in CoL sp. nov., is here described for the Western Pacific species. Uca jocelynae View in CoL sp. nov. has not been recorded from Australia and New Caledonia as yet, but another member of the U. vocans View in CoL complex, U. vomeris View in CoL , is known from these two localities ( Crane 1975; this study). Uca neocultrimana therefore appears to be restricted to Fiji ( Bott 1973; Crane 1975; Barnwell 1982) and eastwards ( Wallis: Poupin 2008; Samoa: Crane 1975) ( Fig. 1 View FIGURE 1 ). To the east of Samoa, however, only U. crassipes View in CoL and U. tetragonon View in CoL have been recorded from French Polynesia thus far ( Poupin 1996).
Like U. neocultrimana , U. jocelynae View in CoL sp. nov. is only known from more oceanic islands and there is no record of either species from continental Asia (e.g. China, Indochina, Sundaic and Sahulian Southeast Asia) and Australia. In these areas, the dominant species of the U. vocans View in CoL complex are U. borealis View in CoL , U. vocans View in CoL , U. dampieri View in CoL and/or U. vomeris View in CoL ( Crane 1975; George & Jones 1982; Dai & Yang 1991). However, in Taiwan and the Ryukyus, U. jocelynae View in CoL is sympatric with the U. borealis View in CoL or U. vocans View in CoL (see Crane 1975; Yamaguchi 1994; this study).
Uca jocelynae View in CoL sp. nov. has been incorrectly identified as U. borealis Crane, 1975 View in CoL , in Taiwan (see Ho et al. 1993; Jeng 1998; Shih 1998; Lee 2001; Ng et al. 2001). This is probably because only U. borealis View in CoL was reported by Crane (1975) and the later authors have merely followed it (see Ng et al. 2001). Crane (1975), however, only examined specimens collected from muddy flat habitats in northwestern Taiwan (Danshuei, Taipei County), where only true U. borealis View in CoL occurs ( Shih 1994).
Shih et al. (2009) studied the genetic differences (16S rRNA and COI) among the species within the Uca lactea View in CoL complex and supported the identity of Uca iranica Pretzmann, 1971 View in CoL , and U. albimana (Kossmann, 1877) View in CoL from the Persian Gulf and Red Sea, respectively, which recognizes six species in the U. lactea View in CoL complex. Davie et al. (in press) also described a new species of soldier crab of the genus Mictyris (Mictyridae) View in CoL from the Ryukyus with the mean interspecific K2P distance with its closest species of 4.4% of the COI. In our study, the mean interspecific COI K2P distance of U. jocelynae View in CoL sp. nov. is 5.32% with U. neocultrimana , which is 8.7 times greater than the mean intraspecific distance of this new species, 0.61% ( Table 2 View TABLE 2 ). This is even considering the lowest interspecific COI K2P distance of this new species is 4.94% with U. neocultrimana , which is 1.23%, 4.0 times greater than the largest intraspecific distance of this new species. The “barcoding gap” ( Meyer & Paulay 2005; Hubert et al. 2008) between interspecific and intraspecific divergence is sufficient to recognize this as a new species. In any case, the morphological differences as well as the allopatry lend support to recognize U. jocelynae View in CoL as a separate taxon.
It is noteworthy that U. borealis , U. dampieri , U. vocans , and U. vomeris share close haplotypes of the combined 16S rRNA and COI (clade U in Fig. 6 View FIGURE 6. A ), 16S RNA, or COI (data not shown). The four species can, nevertheless be easily separated morphologically (at least for males) by the shape of major chela and the structure of the G1 (see Crane 1975: Fig. 64A–F). Crane (1975), however, treated them only as different subspecies of U. vocans , although her concept of subspecies is not the same as what is used today. A similar case has been reported in a study of the East Asian varunid mudflat crabs of the genera Helice and Chasmagnathus (Shih & Suzuki 2007) . In this study, Helice formosensis Rathbun, 1931 , H. latimera Parisi, 1918 , and H. tientsinensis Rathbun, 1931 , also shared close haplotypes of 16S rRNA and COI, although the number of suborbital tubercles, a diagnostic species character, among them are variable. The identical genetic composition of the above species may be due to their recent speciation, which has produced only minor differences to be resolved by these mitochondrial markers. It cannot be discounted that this may only be due to clinal and/or geographic variations. Further studies by using markers with higher resolution or nuclear markers as well as ecological and behavioral studies may be necessary to clarify their taxonomy.
TABLE 2. Matrix of percentage pairwise nucleotide divergences with K 2 P distance (lower left) and mean number of differences (including gaps) (upper right) based on 526 bp of 16 S rRNA and 658 bp of COI (in parentheses) within and between clades of the Uca vocans complex. Clade U includes the species of U. borealis, U. dampieri, U. vocans, and U. vomeris.
| N u c l e o t i d e divergence | M e a n nucleotide d iff e r e n c e | U. j o c e l y n a e sp. nov. | U. neocultrimana | U. hesperiae | Clade U | |
|---|---|---|---|---|---|---|
| U. jocelynae sp. nov. | 1.80 (4.02) | 0.35 (0.61) | — | 18.2 (33.25) | 2 4. 8 0 (49.92) | 24.97 (47.2) |
| U. neocultrimana | — (4.67) | — (0.72) | 3.41 (5.32) | — | 2 5. 0 0 (51.67) | 27.17 (46.86) |
| U. hesperiae | — (2.00) | — (0.3) | 4.15 (8.16) | 4.39 (8.49) | — | 4.17 (24.29) |
| Clade U | 2.47 (4.67) | 0.41 (0.72) | 4.15 (7.69) | 4.80 (7.64) | 0.77 (3.84) | — |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Uca (Gelasimus) jocelynae
| Shih, Hsi-Te, Naruse, Tohru & Ng, Peter K. L. 2010 |
Uca (Gelasimus) neocultrimana
| Ng 2008: 240 |
Uca vocans
| Minemizu 2000: 311 |
Uca
| Jeng 1998: 95 |
| Shih 1998: 67 |
Uca neocultrimana
| Takeda 1995: 104 |
Uca borealis
| Lee 2001: 102 |
| Ng 2001: 37 |
| Ho 1993: 20 |
Uca vocans vocans
| Tzeng 1992: 163 |
Uca vocans pacificensis
| Yamaguchi 1994: 185 |
| Barnwell 1982: 70 |
Uca (Thalassuca) vocans pacificensis
| Crane 1975: 90 |
Mesuca (Latuca) neocultrimana
| Bott 1973: 317 |
Uca marionis
| Takeda 1973: 18 |